TELMISARTAN as Effective as Ramipril in patients at High Risk for Vascular Events
ONTARGET (Ongoing Telmisartan Alone and in Combination with Ramipril Global End-point Trial)
18 Mar, 14
ONTARGET
Aim
To compare the ACE inhibitor ramipril, the ARB telmisartan, and the combination of the two drugs in patients with vascular disease or high-risk diabetes.
Study Patients
25,620 patients with coronary, peripheral, or cerebrovascular disease or diabetes with end-organ damage.
Telmisartan group = 8542 patients
Ramipril group = 8576 patients
Telmisartan + Ramipril group = 8502 patients
Study Groups
- Telmisartan 80 mg
- Ramipril 10 mg
- Telmisartan 80 mg + Ramipril 10 mg
Study Duration
56 months
Results
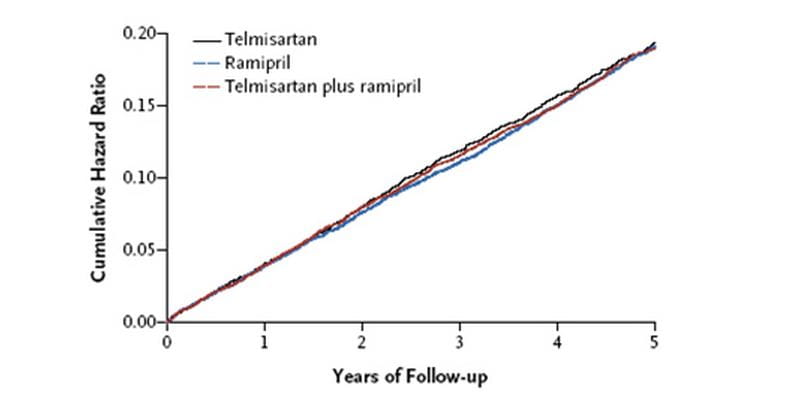
- Telmisartan (16.7%) was as effective as ramipril (16.5%) in reducing the primary composite outcome of death from cardiovascular causes, myocardial infarction, stroke,or hospitalization for heart failure (relative risk 1.01).
- Telmisartan was as effective as ramipril in reducing the secondary endpoint of death from cardiovascular causes, myocardial infarction, or stroke.
- Telmisartan had lower rates of cough (1.1%vs.4.2%) and angioedema (0.1%vs.0.3%) as compared to ramipril.
- No additional advantage from the combination of telmisartan and ramipril used in full doses in this population, as compared with ramipril alone
- Mean blood pressure was lower in both the telmisartan group (a 0.9/0.6 mm Hg greater reduction) and the combination-therapy group (a 2.4/1.4 mm Hg greater reduction) than in the ramipril group.
Conclusion
Telmisartan was equivalent to ramipril in patients with vascular disease or high-risk diabetes and was associated with less angioedema.
N Engl J Med 2008; 358: 1547-59