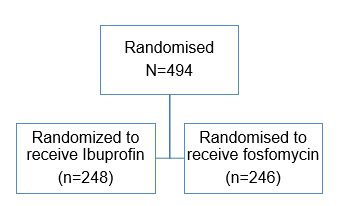
Ibuprofen for Initial Symptomatic Treatment of Uncomplicated UTI
Introduction
Uncomplicated urinary tract infections are common in general practice, where they account for 25% of antibiotic prescriptions. The alternative symptomatic treatment approach and to what extent it can reduce the overall antibiotic prescription rate is unknown. Ibuprofen has demonstrated non-inferiority for symptom resolution, with 24/36 women recovering without antibiotic treatment in a study that was inadequately powered for a definitive result.
Aim
To assess whether the number of antibiotic prescriptions issued for uncomplicated urinary tract infection can be reduced by symptomatic treatment with ibuprofen.
Patient Profile
- N= 1184 women with suspected urinary tract infection were screened
- Women aged 18-65 with typical symptoms of UTI and without risk factors or complications
- Dysuria and/or frequency/urgency of micturition
- With or without lower abdominal pain
- Any signs of upper urinary tract infection (fever, loin tenderness)
- Current conditions that could increase the likelihood of potentially complicated courses (such as pregnancy or renal diseases)
- Urinary tract infection within the past two weeks
- Urinary catheterisation
|
|
Ibuprofen (n=241) |
Fosfomycin (n=243) |
|
Mean (SD) age (years) |
37.3 (14.6) |
37.3 (14.3) |
|
Median (IQR) age (years) |
36.0 (24.0-50.0) |
34.0 (24.0-49.0) |
|
Duration of symptoms at inclusion (days)*: |
|
|
|
<1 |
50 (21) |
49 (21) |
|
1-2 |
104 (43) |
80 (34) |
|
>2-7 |
66 (27) |
82 (34) |
|
>7 |
21 (9) |
28 (12) |
|
Symptoms at inclusion*: |
|
|
|
Dysuria |
224 (93) |
218 (91) |
|
Frequency/urgency |
231 (96) |
232 (97) |
|
Low abdominal pain |
172 (71) |
169 (71) |
|
Mean (SD) symptom severity sum score† |
6.0 (2.2) |
6.1 (2.5) |
|
Median (IQR) symptom severity sum score† |
6.0 (4.0-8.0) |
6.0 (4.0-8.0) |
|
Mean (SD) dysuria score‡ |
2.3 (1.0) |
2.3 (1.1) |
|
Median (IQR) dysuria score‡ |
3.0 (2.0-3.0) |
2.0 (1.0-3.0) |
|
Mean (SD) frequency/urgency score‡ |
2.4 (1.1) |
2.4 (1.1) |
|
Median (IQR) frequency/urgency score‡ |
2.0 (2.0-3.0) |
2.0 (2.0-3.0) |
|
Mean (SD) low abdominal pain score‡ |
1.3 (1.1) |
1.4 (1.1) |
|
Median (IQR) low abdominal pain score‡ |
1.0 (0.0-2.0) |
1.0 (0.0-2.0) |
|
Recurrent UTI§ |
42 (17) |
54 (23) |
|
Mean (SD) activity impairment score¶ |
9.6 (5) |
8.9 (6) |
|
Dipstick results**: |
|
|
|
Leukocytes positive |
205 (85) |
200 (83) |
|
Erythrocytes positive |
180 (75) |
189 (78) |
|
Nitrite positive |
53 (22) |
46 (19) |
|
Culture results: |
|
|
|
Urine culture positive†† |
179/237 (76) |
181/234 (77) |
|
E coli |
143/179 (80) |
142/181 (79) |
|
Proteus mirabilis |
12/179 (7) |
8/181 (4) |
|
Staphylococcus saprophyticus |
8/179 (5) |
8/181 (4) |
|
Enterococcus faecalis |
3/179 (2) |
8/181 (4) |
|
Streptococcus agalactiae |
0/179 (0) |
2/181 (1) |
|
Klebsiella pneumoniae |
3/179 (2) |
1/181 (1) |
|
Other uropathogens |
10/179 (6) |
9/181 (5) |
|
Not specified |
0/179 (0) |
3/181 (2) |
|
Susceptibility to fosfomycin (rate): |
|
|
|
All uropathogens |
168/181 (93) |
162/177 (92) |
|
E coli |
142/143 (99) |
142/142 (100) |
SD=standard deviation; IQR=interquartile range; UTI=urinary tract infection.
*n=239 in fosfomycin group.
†Range 0-12. Sum of daily symptom sum scores of dysuria, frequency/urgency of micturition, and low abdominal pain, each on a five-point scale from 0
(not at all) to 4 (very strong/frequent).
‡Range 0-4
§UTI within the past year.
¶Activity impairment assessment, sum score range 0-20.
**n=242 in fosfomycin group.
††Bacterial count >102 cfu/mL.
Methods
- A double-blind randomized multicentre comparative effectiveness trial
- Patients were randomly assigned to the treatment or control groups
- Computerized random number generator was used to carry out randomization in blocks of six in a 3:3 ratio
Study Drug
- Ibuprofen tablets (3×400 mg daily for three days plus 1×1 sachet placebo granules)
- Fosfomycin- trometamol (1×3 g sachet plus 3×3 placebo tablets for three days)
Endpoints
Primary endpoint
- The total number of courses of antibiotics on days 0-28 (for urinary tract infection or other conditions)
- The burden of symptoms on days 0-7, measured as the area under the curve of the sums of daily symptom scores
- The symptom score included dysuria, frequency/urgency of micturition, and low abdominal pain, each on a five-point scale from 0 (not at all) to 4 (strong/frequent)
Secondary outcomes
- The numbers of severe adverse events, complications (febrile urinary tract infection, pyelonephritis, septic syndrome)
- All adverse events, relapses (recurrent urinary tract infection after initial resolution of symptoms) up to day 28 and within six and 12 months,7 and women without symptoms at days four and seven (defined as a symptom sum score of 0)
- Symptom load until day four and symptom load with regard to specific symptoms until day seven
- Assessment of activity impairment on days 1-7
- The number of daily defined doses of antibiotics per patient
Results
- Initial symptomatic treatment with ibuprofen reduced the overall number of antibiotic treatment courses in women with uncomplicated urinary tract infection by 67%, compared with immediate antibiotic treatment with fosfomycin
- Significantly more women received prescriptions of antibiotics in the follow-up period (21.2%), the total number receiving antibiotics was lower in the ibuprofen group by 64.7%
|
|
Ibuprofen (n=241) n(%) |
Fosfomycin (n=243) n(%) |
P value |
|
Primary endpoints |
|
|
|
|
Women who received antibiotics: |
|
|
|
|
Total |
85 (35) |
243 (100) |
<0.001 |
|
By randomisation |
0 (0) |
243 (100) |
- |
|
During follow-up (all)* |
85 (35) |
34 (14) |
<0.001 |
|
During follow-up (for UTI) |
75 (31) |
30 (12) |
<0.001 |
|
Mean (SD) symptom burden day 0-7† |
17.3 (11.0) |
12.1 (8.2) |
<0.001 |
|
Secondary endpoints |
|
|
|
|
Adverse events in patients: |
|
|
|
|
Patients reporting serious adverse events‡ |
4 (2) |
0 (0) |
0.06 |
|
Serious adverse events probably drug related |
1 (0.4) |
0 (0) |
0.32 |
|
Patients reporting adverse events‡ |
42 (17) |
57 (24) |
0.12 |
|
Relapses/complications: |
|
|
|
|
All recurrent UTI until day 28 |
27 (11) |
34 (14) |
0.41 |
|
Early relapse of symptoms (up to day 14)§ |
13 (5) |
7 (3) |
0.18 |
|
Recurrence of UTI (day 15-28)§ |
14 (6) |
27 (11) |
0.049 |
|
Pyelonephritis§ |
5 (2) |
1 (0.4) |
0.12 |
|
Febrile UTI/ (day 0-7)§ |
3 (1) |
0 |
0.12 |
|
Worsening symptoms (day 0-7)§ |
8 (3) |
5 (2) |
0.42 |
|
Patients without symptoms day 4§ |
91/234 (39) |
129/229 (56) |
<0.001 |
|
Patients without symptoms day 7¶ |
163/232 (70) |
186/227 (82) |
0.004 |
|
Mean (SD) symptom duration after randomisation (days) |
5.6 (2.2) |
4.6 (2.2) |
<0.001 |
|
Mean (SD) symptom burden day 0-4† |
13.1 (7.1) |
10.1 (5.9) |
<0.001 |
|
Mean (SD) symptom burden with regard to dysuria day 0-7 |
6.8 (4.6) |
4.5 (3.6) |
<0.001 |
|
Mean (SD) symptom burden with regard to frequency/urgency day 0-7 |
6.5 (4.1) |
4.6 (3.4) |
<0.001 |
|
Mean (SD) symptom burden with regard to low abdominal pain day 0-7 |
4.1 (4.3) |
2.9 (3.1) |
0.001 |
|
Mean (SD) activity impairment assessment day 0-7 |
30.3 (24.5) |
19.5 (16.7) |
<0.001 |
SD=standard deviation; UTI=urinary tract infection.
*Including antibiotic prescriptions for other reasons—for instance, acute bronchitis, otitis.
†Defined as the area under curve (AUC) of daily symptom sum scores day 0-7.
‡As rated by patients.
§As rated by general practitioners.
¶Symptom free is defined as symptom sum score=0.
- The total symptom burden (area under the curve) observed on days 0-4 in the ibuprofen group was significantly higher than in the fosfomycin group.
- Women in the ibuprofen group showed slightly higher scores in impairment of activity
- On day seven, 2% of the women overall still felt impaired most or all the time
- A subgroup analysis stratified for urine culture results in the ibuprofen group showed significant differences in both co-primary outcomes
- women with negative results showed significantly lower symptom burden
- antibiotic use was significantly more reduced in women with positive culture results
- The overall rates of adverse events were comparably low in both groups
- Four serious adverse events occurred that lead to hospital referrals one of these was potentially related to the trial drug
- There were more cases of pyelonephritis in the ibuprofen group, and the same trend was observed in worsening symptoms, febrile urinary tract infections, and symptoms of early relapse (up to day 14)
- The pyelonephritis rate in the ibuprofen group was comparably high (2.1%)
Conclusion
- The hypothesis of non-inferiority of ibuprofen initial symptomatic treatment with was rejected
- Significant reduction in antibiotic use was observed with initial treatment with ibuprofen in women aged 18-65 with mild to moderate symptoms of urinary tract infection but was less effective for symptom relief, and there were more cases of pyelonephritis
- Based on the findings the study authors recommend that this treatment regimen can be discussed with women who are willing to avoid antibiotics or to accept a delayed prescription rather than to all those with an uncomplicated UTI.
BMJ 2015;351:h6544