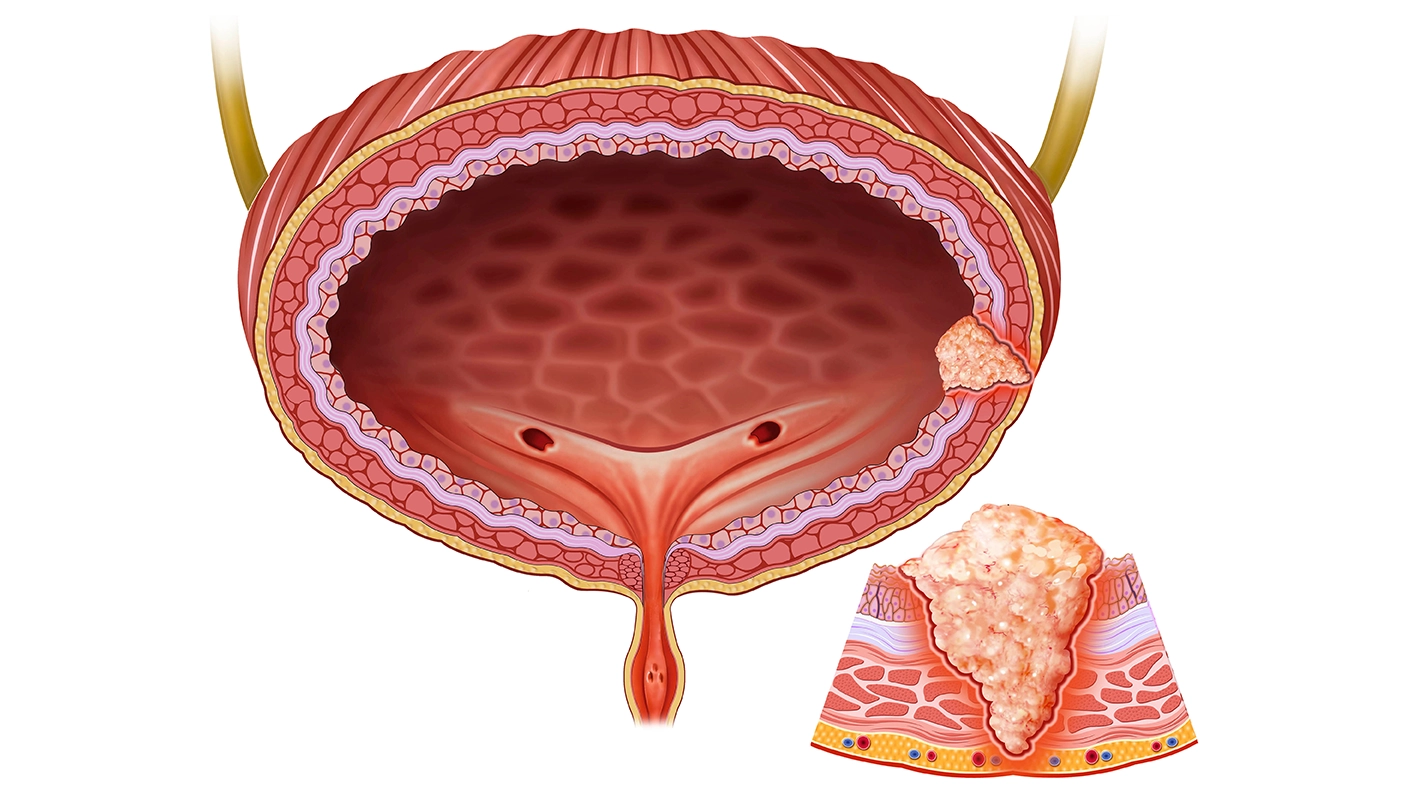
No Need for TURB, VI-RADS Makes the Diagnosis by Prof. V. Panebianco
The session discusses bladder cancer complexities, with a focus on bladder cancer diagnosis and management. Traditionally, diagnosis of bladder cancer involves hematuria detection, followed by ultrasound, cytology, cystoscopy, and Transurethral Resection of Bladder Tumour (TURBT) staging. However, TURBT limitations include operator dependence, low sensitivity, residual disease (58%), lack of sense of the tumour deepness (T3 stage), incomplete mapping accuracy, and costs linked to bladder cancer. A decade ago, TURBT led to understaging of 48% of tumors, impacting the survival of patients. A RESEQ study revealed variations in TURBT quality globally and within regions, with recurrence rates differing between sites, independent of tumor factors.
Magnetic Resonance Imaging (MRI) and Vesical Imaging-Reporting and Data System (VI-RADS) have emerged as potential tools to improve bladder cancer management. VI-RADS, now part of the American College of Radiology, offers a standardized approach to assessing muscle invasion in bladder cancer. The high contrast resolution of MRI facilitates precise staging and differentiation of tumor layers, thereby assisting in treatment decisions, particularly by distinguishing between the inner layer and the muscularis propria. Numerous studies on PubMed have showcased the diagnostic efficacy of VI-RADS, providing evidence at levels 2A and 2B.
A new concept of MRI for bladder mapping is proposed, suggesting that patients with hematuria, positive cytology, and vegetating lesions on ultrasound, along with VI-RADS 2 findings on MRI, could undergo TURBT for therapeutic intent only. This approach advocates for a non-muscle invasive bladder cancer (MIBC) MRI pathway, emphasizing the importance of histoscopy replacement by MRI and avoiding unnecessary TURBT among high-risk T1 patients. In MIBC cases categorized as VI-RADS 1 and 2, TURBT is recommended solely for therapy, eliminating the need for histoscopy before therapeutic procedures and reducing unnecessary invasive interventions. Conversely, for cases classified as VI-RADS 3 and 4, MRI can aid in confirming staging, with the potential for biopsy confirmation in VI-RADS 5 cases involving an extension to adjacent tissues. This approach not only enhances the accuracy of staging but also minimizes the risk of understaging, thus optimizing patient outcomes. MRI pathways for bladder cancer are supported by literature and Delphi Consensus results. The recommendation emerged that MRI should precede treatment, leading to the introduction of guidelines advocating for MRI before TURBT, although the current strength of ratings is weak.
Therefore, MRI is crucial for accurate tumor staging and differentiation. Personalized care is vital to prevent tumor understaging. Limiting TURBT to therapeutic use reduces unnecessary procedures. However, challenges related to MRI expertise, scanner availability, detection of carcinoma in situ (CIS), and histological variants persist. Technology like photon counting CT and AI-assisted software for histological variant discrimination can be used.
TURBT is Mandatory Before any Treatment by Dr Antoine G
In contrast to the typical cancer diagnostic approach utilizing a single biopsy alongside imaging, bladder cancer diagnostics follow a distinct procedure. The standard procedure involves excising the exophytic tumor part and the underlying bladder wall, a technique known as TURBT, first described by Edwin Beer in 1910. The advent of electrocoagulation via cystoscopy revolutionized bladder tumor treatment. Imaging in bladder cancer diagnosis aims to evaluate tumor extent, lymphatic spread, and distant metastasis, with Computed Tomography (CT) serving as the primary technique due to its widespread availability, ease of use, high resolution, and short acquisition time. However, CT's limitation lies in its inability to differentiate between T1 and T3a tumors. It is only reliable in detecting invasion into perivascular fat tissue or adjacent organs, with an accuracy ranging from 55% to 92% for identifying extravesical tumor extension.
TURBT is essential for neoadjuvant chemotherapy (NAC) and trimodality treatments. NAC, particularly cisplatin-based, is recommended for muscle-invasive urothelial carcinoma patients who are fit for cisplatin, while variant histologies like squamous cell carcinoma or adenocarcinoma may permit immediate cystectomy. Trimodality treatments typically commence with a comprehensive TURBT. A study published in 2012 in European Urology showed that patients undergoing radical TURBT exhibit higher complete response rates and better survival outcomes compared to those without radical TURBT (79% vs 57%), underscoring the significance of thorough tumor resection in bladder cancer management.
There are drawbacks associated with TURBT which include treatment delays, potential release of circulating tumor cells, and staging errors. Preliminary data from the bladder PATH trial indicated a 45-day reduction in time to definitive treatment for patients with MIBC when MRI was used instead of TURBT. Additionally, TURBT may release circulating tumor cells into the bloodstream, potentially leading to metastasis and treatment resistance, as demonstrated in a small retrospective study. Moreover, staging errors are a concern, with upstaging observed in 35% of T1G3 bladder cancer cases managed by immediate cystectomy. This may be due to difficulties in recognizing detrusor muscle in the specimen or inadequate resection. To address these issues, a randomized controlled trial is underway in the Netherlands comparing standard TURBT with multiparametric MRI (mpMRI) plus single bladder biopsy for suspected MIBC. However, until these results are available, TURBT remains essential for diagnosis and management.
FDG PET/CT for Staging and Restaging of MIBC by Prof Shahrokh F. Shariat
The current standardized treatment for bladder cancer, involving radical cystectomy (RC) and neoadjuvant chemotherapy (NAC) with random template lymph node dissection (LND), has resulted in poor overall survival rates of 50-60% at five years due to missed micrometastasis and over-treatment, leading to adverse effects. Undertreatment is also a concern, with false assessments of metastatic status, and a tendency for localized therapies after neoadjuvant treatment may miss micrometastasis. Minimally invasive surgery may lead to inadequate tissue margins and missed lymph nodes. Thus, personalized medicine, tailored to tumor characteristics and patient needs, is essential to improve outcomes and reduce treatment-related complications.
To know the potential role of FDG PET/CT as a biomarker for personalized treatment in cancer management a hypothesis posited was that 2-deoxy-2-[fluorine-18]fluoro- D-glucose integrated with computed tomography (FDG PET/CT) could significantly alter clinical decision-making, thereby improving patient outcomes by minimizing both over and under-treatment. It was suggested that FDG PET/CT could enhance lymph node and distant staging and aid in patient reclassification, particularly after neoadjuvant therapy. However, uncertainty exists regarding its efficacy in lymph node staging due to limited evidence. While FDG PET/CT shows comparable specificity to contrast-enhanced CT, it still yields false positives in 8% of cases, potentially leading to overtreatment. Additionally, its sensitivity, ranging from 50-60%, is considered inadequate for preventing overtreatment. Further research is needed to optimize FDG PET/CT utility in treatment decision-making. A study conducted by Martins and her team, involving over 500 patients, revealed that FDG PET/CT had a lower sensitivity of 23% compared to contrast-enhanced CT. Despite potential biases in the study, it suggested that FDG PET/CT may detect more micrometastatic diseases missed by contrast-enhanced CT, with these missed metastases likely being smaller in size.
There is a potential for improving outcomes in FDG PET/CT by addressing challenges related to positivity criteria. It can be done by modifications such as considering the actual diameter of lymph nodes and adjusting the cutoff of maximum standardized uptake value (SUV max), which has shown promise as a prognostic marker but may reduce sensitivity. To assess the impact of these changes on clinical decision-making a study involving over 711 consecutive patients who underwent retrospective PET CT analysis showed that approximately 26% of patients had changes in their clinical stage, while the recommended strategy in clinical management shifted for 18% of patients. While these changes were significant, they also led to false positives in approximately 1 in 12 patients, resulting in over-treatment with associated harms.
The response to NAC is being studied to determine bladder sparing eligibility and the need for intensified therapy or surgery. Limited data from a meta-analysis of five studies involving 278 patients suggests improved lymph node sensitivity, but the overall evidence is retrospective. Other systemic therapies like PEMBRO in the neoadjuvant setting show less favorable results. PET MRI's efficacy is uncertain due to limited data, but it may offer improved specificity for local tumors. Guidelines regard FDG PET/CT as supplementary information, with the National Comprehensive Cancer Network (NCCN) suggesting potential usefulness and NICE guidelines offering slightly more favorable views, especially for high-risk patients with indeterminate findings on CT or MRI.
FDG PET/CT faces challenges including mediocre performance, false positives, urinary excretion issues, lack of standardization, financial burden, and radiation exposure. Promising future tracers include fibroblast activation protein, nectin 4, and PD-L1 targeting agents, which hold the potential for guiding therapy. While PET tracers improve management and may guide targeted therapy, their sensitivity remains insufficient compared to contrast-enhanced CT. They are valuable for clarifying ambiguous cases but do not universally alter treatment strategies, thus not yet mandatory.
Histological Subtypes, Molecular Phenotype, and Biomarkers are Enough by Pr Eva Comperat
The International Collaboration of Cancer Reporting (ICCR) is recognized as the standard for cancer reporting. The cystectomy reports must detail resections, tumor subtype, presence of noninvasive carcinoma, grade, micro/macrovascular invasion, co-existing pathology, and ancillary studies. Resection reports should also cover tumor size, margins, lymph nodes, and pTNM. Sample size, tumor percentage, invasive carcinoma presence, microscopic extension, lymphovascular invasion, and coexisting pathologies are essential. Pathological Tumor-Node-Metastasis (pTNM) should be included, with "P" for cystectomy and "T" for resection. These elements form the reporting framework.
The importance of histological subtypes in bladder cancer is emphasized, with distinctions drawn between muscle-invasive and non-muscle-invasive subtypes. The significance of these subtypes lies in diagnostic considerations, prognosis, and therapeutic implications. Some subtypes, such as micropapillary, are associated with more aggressive behavior. Studies have shown that pure urothelial carcinoma tends to have better outcomes compared to subtypes. Pathology reports provide crucial information for treatment and staging decisions. However, advancements in molecular phenotyping have introduced new insights into bladder cancer subtypes. While there is an attempt to integrate surgical pathology with molecular pathology, it is not always successful. Despite known associations between certain variants and subtypes, molecular testing is not universally recommended, especially by pathology societies. It is noted that RNA variant examination provides a more precise profile compared to immunohistochemistry for molecular phenotyping.
In the microscope, subtypes such as the large nested variant are easily recognizable. Even if not requested by surgeons or urologists, testing is initiated as these subtypes often exhibit FGFR mutations, for which specific treatments are available. The primary concern is identifying who would respond well to NAC, for which there currently no definitive marker exists. Variants of the molecular part may offer potential insights, with studies suggesting that tumors displaying genetic instability (blue line or green line tumors) exhibit an enhanced response to NAC. Interestingly, this response is observed independently of the clinical stage, particularly evident in cases of UroC or GU tumors, which demonstrate markedly improved outcomes following NAC. Genetically unstable variants exhibit superior pathological responses and greater downstaging. Hence, variant pathology holds considerable importance and has been shown to respond favorably to specific biomarkers.
When further advancing in staging, it is noted that different variants yield different types of metastases. For instance, urothelial carcinomas commonly result in lymph node and bone metastases, while squamous carcinomas tend to metastasize to the lung and liver. The importance of molecular subtyping is underscored in predicting the occurrence, timing, and location of metastases. Biomarkers play a crucial role in staging, with urinary cytology primarily detecting high-grade lesions. Other biomarkers, such as tissue-based biomarkers like PD-L1 and HER2, genetics like FGFR, and liquid biopsies, offer the potential for detecting low-grade lesions, although their reliability may vary based on patient age and treatment history. Histology remains significant, but the distinction between morphology and molecular class in defining urothelial carcinoma is still evolving. The approach to treating classical and subtype histologies is uncertain, highlighting the need for further research and the expanding role of biomarkers and molecular profiling in cancer management.
MRI in Evaluation after Neoadjuvant Chemotherapy by Andrea Necchi, MD
In this session, each feature of the multi-parametric MRI was assessed for disease detectability, using a binary yes or no response. Complete response was defined as no detection across all features. The moderator then presented results regarding the prediction of complete response. The challenge identified was the need to detect outliers and predict outlier responses, especially in a curative setting, potentially sparing patients from cystectomy. The potential utilization of this tool to avoid cystectomy or spare the bladder for these patients is being considered. It is suggested that more research needs to be conducted in the overall management to assess how residual disease, such as pTa residual disease, may impact post-treatment patient outcomes and treatment options, including cystectomy. Additionally, the possibility of reducing patient discomfort by applying a pre-parametric MRI, as done in prostate cancer, is being explored. The accuracy of this approach, particularly in the PURE-01 cohort, appears promising, although larger validation studies are necessary. The integration of PET CT as a fourth parameter, despite limitations in lymph node detection, is due to uncertainty regarding its interpretation in bladder tumor imaging.
The algorithm for nacVI-RADS, which was developed by the Valeria Group, has been found to be quite promising, although its validation in a larger immunotherapy setting is currently underway. The introduction of the VI-RADS ‘0’ category aims to modify the VI-RADS score, indicating the absence of residual disease. This is crucial due to limitations in QRBT, necessitating clear staging, particularly in patients undergoing neoadjuvant therapy. There is a consistent patient population, starting from VI-RADS 4 and 5 categories, who show downstaging to VI-RADS less than or equal to 3, with a response rate of 30% to neoadjuvant immunotherapy. The paradigm is shifting from a therapeutic perspective in these cases.
Several trials testing various neoadjuvant therapies are underway. For instance, Sacituzumab govitecan, a novel ADC, is being tested prior to cystectomy. In this case, Vi-RADS 5 was observed pre-therapy, with no response evident in the post-therapy images, corresponding to a PT3 disease RC. Another case pertains to the NeuroCombo study, which evaluates the combination of nivolumab and Abraxane as neoadjuvant immunotherapy. Pre-therapy images showed VI-RADS 5 lesions, with a complete histological response observed post-treatment, corresponding to a VI-RADS 0 response. Furthermore, a study is examining the efficacy of Epacadostat, a serotonin agent inhibitor where patients initially presented with VI-RADS 1 lesions, and following TURBT were downstaged to non-muscular invasive disease. However, some patients exhibited non-response or even progressive disease, as indicated by VI-RADS 2 or VI-RADS 5 lesions post-treatment, respectively, ultimately corresponding to PT1 disease after rc.
The PURE-01 study suggests a potential alignment between Valeria and Eva's perspectives, revealing an association between VI-RADS scores and gene expression profiling. Lower VI-RADS scores 0-3 correlate with higher immune gene signature scores and lower angiogenesis scores, implying a biological basis beyond clinical staging. However, the focus remains on defining a clinical complete response to guide post-therapy decision-making, impacting the need for cystectomy or bladder-sparing approaches. VI-RADS proves prognostic post-therapy across various cohorts, emphasizing its importance in patient management. Further investigation, including exploring biomarker tools like circulating tumor DNA, is urged to address uncertainties surrounding treatment response assessment and cure definition in MIBC, encompassing options such as RC and chemoradiation.
European Association of Urology (EAU) Annual Congress 2024, 5th April - 8th April 2024, Paris, France