ENERGIZE-T: A Global, Phase 3, Double-Blind, Randomized, Placebo-Controlled Study of Mitapivat in Adults with Transfusion-Dependent Alpha- or Beta-Thalassemia
Speaker: Maria Cappellini, University of Milan, Italy
Key Highlights
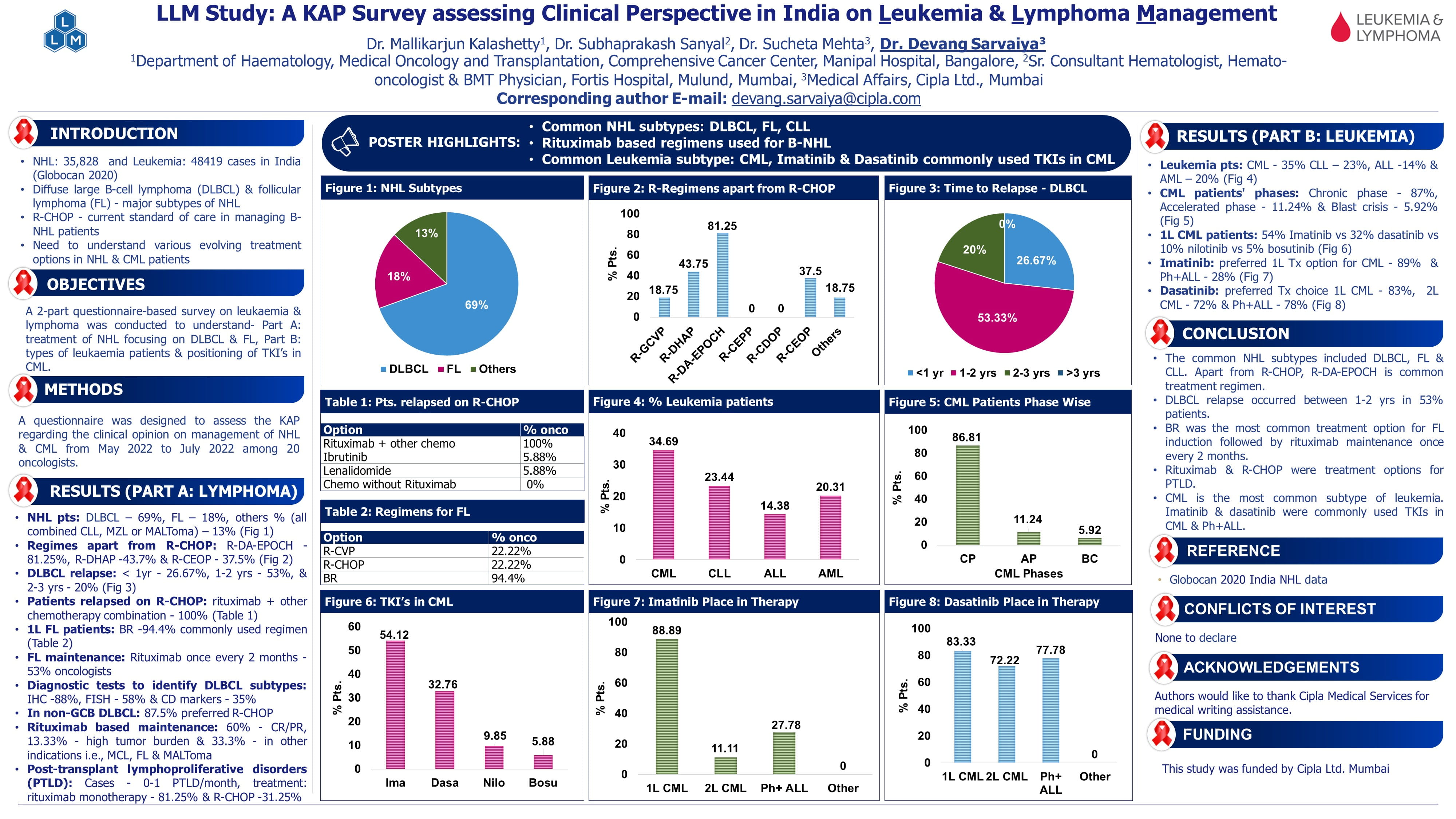
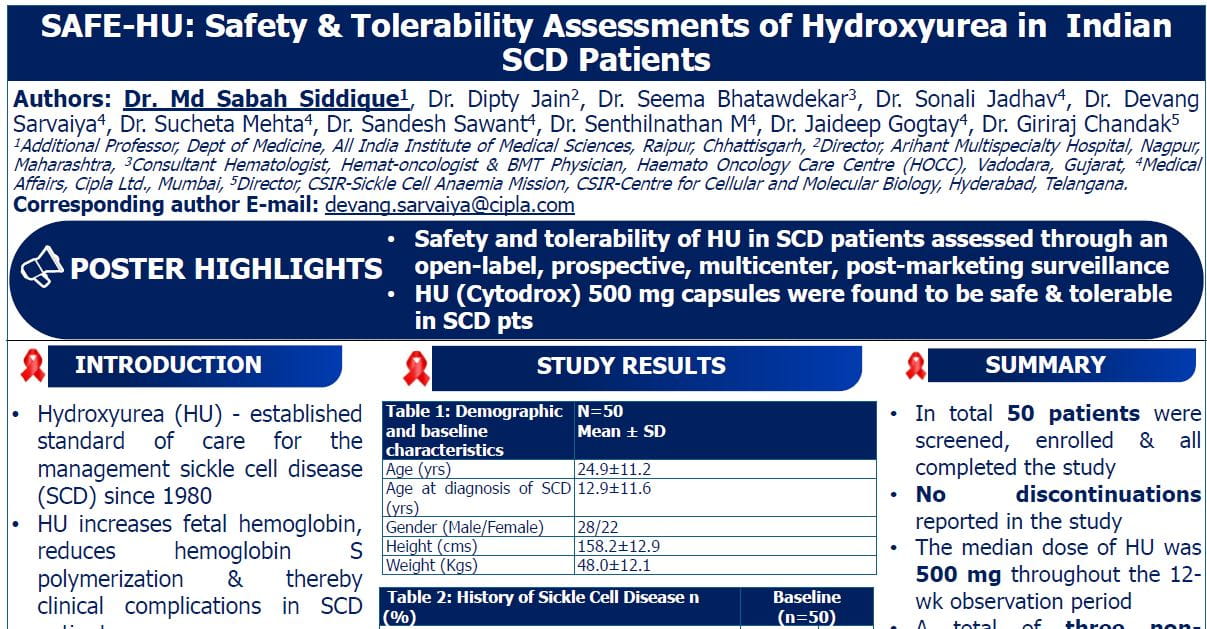
Dr. Maria Cappellini presented findings from the ENERGIZE-T trial, a global, Phase 3, double-blind, placebo-controlled study evaluating Mitapivat, a pyruvate kinase (PK) activator, in adults with transfusion-dependent (TD) alpha- or beta-thalassemia. This trial aimed to assess the efficacy and safety of Mitapivat in reducing transfusion burden.
Study Background and Rationale
-
Thalassemia Burden: Despite advances in care, transfusion-dependent thalassemia (TDT) patients face significant morbidity due to anaemia, transfusion burden, and iron overload.
-
Mitapivat’s Mechanism: As a PK activator, mitapivat enhances ATP production in red blood cells (RBCs), improving cell energy and reducing oxidative stress and membrane damage. Prior studies demonstrated its efficacy in non-TDT, improving haemoglobin and patient well-being.
Study Design
-
Patient Cohort: The study included a unique subgroup who were previously underrepresented in other studies. These patients had specific clinical features that warranted focused attention to ensure their inclusion and response monitoring.
-
Randomization and Treatment Regimen: Patients were randomized in a 2:1 ratio:
-
2: Mitapivat (100 mg twice daily)
-
1: Placebo
-
The double-blind period lasted 48 weeks.
-
At the end of this period, patients on placebo could switch to mitapivat as part of an open-label extension for an additional 5 years.
-
Eligibility Criteria
-
Adults aged >18 years.
-
Required a clear diagnosis of transfusion dependency, defined as requiring 6 to 20 units of blood within 24 weeks before enrolment.
-
Exclusion Criteria:
-
Patients on luspatercept.
-
History of gene therapy or hemopoietic stem cell transplant exposure.
-
Stratification during Randomization: Patients were stratified based on:
-
Genotype: β0/β0 vs Non-β0 /β0.
-
Geographical region: Northern America, Europe, Asia Pacific, and other regions globally.
-
Study Endpoints
-
Primary Endpoint
-
Transfusion Reduction Response: Defined as:
-
≥50% reduction in red blood cell transfusion units compared to baseline during the 48-week study period.
-
Key Secondary Endpoints
-
Transfusion reduction by ≥50% or ≥33% over 24 weeks.
-
Additional periods were analyzed from week 13 to week 48 for sustained response.
-
Additional Efficacy Analysis
-
Transfusion Independence: Defined as patients being free from any transfusion for ≥8 consecutive weeks during the 48 weeks.
Patient Characteristics
-
Cohort Selection
-
Total number of screened patients: 305
-
Randomized patients: 258
-
Allocated to mitapivat: 171 patients
-
Allocated to placebo: 87 patients
-
Demographic Overview:
-
Age and gender distribution were similar.
-
The majority of participants were White or Asian.
-
Thalassemia Genotype: Balanced across groups with categories beta 0 vs. non-beta 0.
-
Pre-Transfusion Hemoglobin Levels: Comparable between the two groups, highlighting similarity in disease severity before intervention.
-
Transfusion Requirements: Larger numbers of patients across both groups required >12 units of transfusion prior to the study.
Results
Efficacy
-
Primary Endpoint: 30.4% of patients in the mitapivat group achieved ≥50% transfusion reduction versus 12.6% in the placebo group (p=0.0003).
-
Key Secondary Endpoints
Statistically significant reductions in transfusion burden were observed for all secondary endpoints:
-
≥50% reduction in transfused RBC over 24-week period through week 48
-
≥33% reduction over fixed periods (weeks 13–48).
-
≥50% reduction in transfused RBC from weeks 13-48
-
Transfusion Independence: 12% of mitapivat-treated patients achieved ≥8 consecutive weeks transfusion-free, with 3 patients remaining transfusion-free through week 48.
-
Higher proportion of patients in the mitapivat group achieved transfusion independence vs placebo.
-
Subgroup Analysis: Reduction in transfusion burden was consistent across all subgroups, including geographical region, age, and sex.
Safety
-
Mitapivat was well-tolerated, with adverse events (AEs) consistent across treatment groups.
-
Common AEs: Upper respiratory infections, diarrhea, fatigue, and insomnia, mostly mild and manageable.
-
No significant AEs leading to treatment discontinuation were reported.
Conclusion
Reduction in transfusion burden was consistent across all subgroups, including treatment with mitapivat, a disease-modifying therapy, was effective in significantly reducing transfusion burden in a globally representative population of TDT patients, including both alpha and beta variants. This impact was consistent across diverse groups and supported by previous trials, including those involving non-transfusion-dependent thalassemia patients.
ASH Annual Meeting and Exposition, 7-10 December 2024, San Diego, California