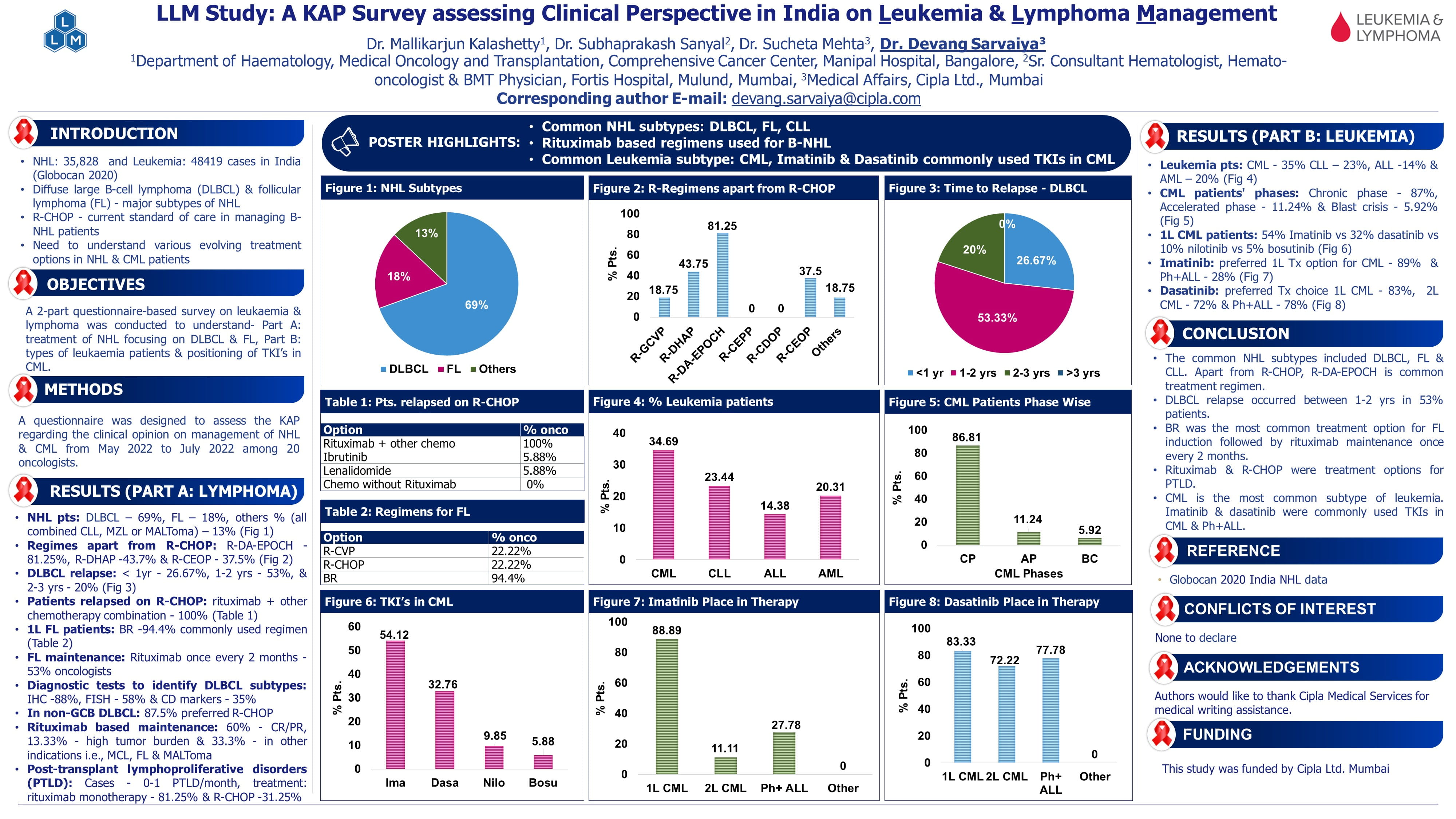
Asciminib (ASC) Demonstrates Favourable Safety and Tolerability Compared with Each Investigator-Selected Tyrosine Kinase Inhibitor (IS TKI) in Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP) in the Pivotal Phase 3 ASC4FIRST Study: Week 96 Update
Speaker: Jorge Cortes, Georgia Cancer Centre, Augusta
Key Highlights
The high selectivity of asciminib, a tyrosine kinase inhibitor (TKI) specifically targeting the ABL myristoyl pocket, makes it a strong candidate for frontline therapy, given its previously demonstrated efficacy in multi-refractory patients. The primary analysis, which led to its approval in the US and ongoing approvals globally, showed significant benefits in efficacy and safety. Today, we present key secondary endpoint results at week 96 of the study.
Study Design and Primary Endpoints:
- The ASC4FIRST trial is a pivotal phase 3 study evaluating the safety and efficacy of asciminib in newly diagnosed chronic myeloid leukaemia in chronic phase (CML-CP). Patients were randomised to asciminib or investigator-selected frontline TKIs (imatinib or second-generation TKIs).
- The primary endpoints included the major molecular response (MMR) at week 48 comparing asciminib to imatinib and to investigator-selected TKI.
- This presentation focused on the 96-week data, including these two endpoints and safety findings.
Patient Characteristics:
- The two groups in this study were well balanced in terms of baseline demographics and risk factors. The median age was in the fifties.
- Around 25-30% of patients had high-risk Framingham scores, while 10-11% had high-risk ELTS scores, and 25-30% had high-risk SOCAL scores.
- Patients in the imatinib strata were older and had higher Framingham scores.
- Patients in the second-generation TKI strata had a higher proportion classified as high-risk ELTS.
- Discontinuations from the study were significantly lower in the asciminib arm (17.9%) compared to the control arm (38.2%). Discontinuations occurred due to adverse events (AE) and unsatisfactory therapeutic effect, with the control arm showing double the rate of these issues compared to asciminib.
Key Secondary Endpoints:
The analysis highlights the efficacy of asciminib at 96 weeks compared to other tyrosine kinase inhibitors (TKIs) and imatinib.
- Major Molecular Response (MMR)
- 96-week data shows that asciminib maintains superiority over other TKIs and imatinib.
- The difference in MMR rates has increased over time in favour of asciminib.
- Asciminib vs. Imatinib: Continued benefit of asciminib in achieving MMR compared to imatinib.
- Asciminib vs. 2nd Generation TKIs: Although this comparison wasn't statistically powered, asciminib showed nearly double the efficacy compared to second-generation TKIs.
- Deep Molecular Responses (MR4 & MR4.5): Deep molecular responses are significantly higher with aciminib:
- ~50% achieved MR4 by week 96.
- ~30% achieved MR4.5.
- These rates are better than other TKIs.
- Overall Population Analysis: Across the entire study population, asciminib showed an increasing difference in MR4 and MR4.5 rates compared to investigator-selected TKI therapies.
Sequential Mutation Analysis:
- Mutations in Aciminib Treatment Group:
- All mutations identified in patients receiving asciminib are myristoyl pocket mutations.
- No mutations were observed in the ITP binding pocket or other regions.
- Resistance Mechanisms:
- Upon emergence of resistance, patients were transitioned to traditional TKIs.
- Many maintained therapy and remained in a chronic phase, though a few experienced accelerated phase transformations.
- Case Example: A patient transitioned to ponatinib, entered chronic phase, but later died following transplantation.
- Control Arm Findings (Imatinib/Nilotinib):
- Emergence of traditional mutations in the HB binding pocket was noted.
- Shift in inhibitors and changes in patient status were documented post-mutation.
Safety Profile:
- AE: Higher rates of AE were observed with imatinib and 2nd-generation TKIs compared to asciminib.
- Discontinuation Rates: Fewer patients discontinued asciminib due to AE (5%) compared to imatinib (10%) and 2nd-generation TKIs (13%).
- Adjustments and Interruptions: More frequent with imatinib and 2nd-generation TKIs compared to asciminib.
Conclusion:
In conclusion, the extended follow-up continues to demonstrate the superiority of asciminib in terms of efficacy compared to all other approved TKIs for frontline CML therapy.
Key highlights:
- Major molecular response rates have risen above 75%, with deep molecular responses nearing 50% in patients treated with asciminib.
- The efficacy differences favoring asciminib have expanded when compared to both imatinib and other TKIs.
- Asciminib maintains a favorable safety profile, with most adverse events similar or lower than those observed with other TKIs.
- Adjustments in treatment interruptions and AE have been significantly less frequent with asciminib.
Update of the Ascend-CML Study of Frontline Asciminib
Speaker: David Yeung, University of Adelaide, Australia
Key Highlights
Asciminib is a first-in-class ABL1-specific inhibitor targeting the ABL1 kinase pocket and is approved for use in multiple jurisdictions, primarily in the third-line setting based on supportive clinical data. Importantly, asciminib has now also been approved for use in the frontline setting in the U.S., following findings from studies like ASC4FIRST and ASCEND.
Ascend-CML Study:
- Cohort Size: 101 patients
- Inclusion Criteria: Adults with good organ function, ECOG 0-2, measurable BCR-ABL transcript.
- Treatment: Asciminib at 40 mg BID.
- Response Monitoring: Patients were assessed against ELN molecular targets (EMR at 3 months, MMR at 12 months, and MR4 at 18 months).
- The patient cohort was predominantly male, Caucasian, diagnosed in their 6th decade of life, and classified as low-risk ELTS.
Efficacy Data:
The report highlights long-term efficacy data from a median follow-up of 3 years (minimum of 2 years) in patients treated with asciminib:
- Patient Outcomes: 80 patients remain on asciminib, with no deaths reported (on or off-study).
- Cumulative Incidence of MMR: 87% at 24 months, showing mature and sustained efficacy over time.
- Deep Molecular Responses:
- MR4:
- 50% at 12 months
- 60% at 2 years
- 73% at 3 years
- MR4.5:
- 31% at 12 months
- 47% at 2 years
- 55% at 36 months
- MR4:
Safety Profile:
Safety and Tolerability: Fewer Events Over Time
Early Toxicities: Most AE occurred in the first year, including:
- Cytopenias: Reported primarily in year 1, with no new significant events thereafter.
- Lipase/Amylase Elevations: Frequent initially but declined in subsequent years.
- Liver Enzyme Abnormalities: Predominantly year 1 events.
- Arterial Occlusive Events: Only one case reported—a 73-year-old woman with pre-existing cardiovascular risk factors.
- Blast Crisis: One case of lymphoid blast crisis occurred and was successfully managed with transplantation.
Patients in the Warning Category:
- Patients with No MMR at 12 Months (10 Patients):
- These patients underwent dose escalation, leading to:
- Median log 4 reduction of 0.4 in BCR-ABL levels.
- Most achieved MMR subsequently following the escalation.
- Patients Without Dose Escalation (2 Patients):
- Despite not dose escalating, these two patients still achieved MMR with:
- Median log 4 reduction of 0.2 in subsequent months.
- Patients Without MR4 at 18 Months (31 Patients)
- Dose Escalation (20 Patients):
- 40% achieved MR4 in subsequent months.
- Median log 4 reduction of 0.3 in BCR-ABL levels.
- No Dose Escalation (11 Patients):
- 36% achieved MR4 without dose escalation.
- Median log 4 reduction of 0.1 in BCR-ABL levels.
- Combination Therapy Observations
- 3 Patients: Received combination therapy (2 due to EMR failure).
- Combination therapy was generally limited to these patients due to EMR failure.
- Dose Escalation (20 Patients):
- Specific Case Insights:
- 1 Patient with BCR-ABL > 1% at 12 months (1.1%):
- Received dasatinib added as per protocol.
- Experienced lipase elevation (no clinical sequelae).
- Adjusted to dasatinib monotherapy as BCR-ABL levels approached MMR.
Conclusion:
Dr. Yeung concluded that the ASCEND CML study highlights that a similar monotherapy shows excellent safety with less frequent toxicity over time. Deep molecular response continues to rise, offering potential to maximize participation in treatment-free remission (TFR). The mutation rate was observed at 8%, consistent with historical data from DASISION and ENESTnd, with the most frequent mutations at residues 337 and 244, most of which are salvageable with dasatinib or nilotinib. Furthermore, the study emphasizes that genomic risk stratification could further enhance patient outcomes, with a new study already underway to explore this approach.
Safety and Efficacy of TGRX-678, a BCR: ABL1 allosteric Inhibitor
Speaker: Qian Jiang, Peking University People's Hospital, China
Key Highlights
Study Overview:
The study evaluating TGRX-678, a BCR::ABL1 allosteric inhibitor, was designed as a phase 1/2 clinical trial. The study's aim was to assess the safety, tolerability, pharmacokinetics (PK), and efficacy of TGRX-678 in patients with CML and BCR::ABL1-positive acute lymphoblastic leukemia (ALL).
Key Features of the Study Design
- Phase 1 (Dose-Escalation Cohort):
- The study used a dose-escalation design to determine the maximum tolerated dose (MTD) of TGRX-678.
- Patients received increasing doses of TGRX-678 to evaluate safety and establish the optimal dose for further studies.
- Phase 2 (Dose-Expansion Cohort):
- After determining the appropriate dose in Phase 1, Phase 2 focused on efficacy and further safety evaluation at that established dose.
- The goal was to assess the clinical activity of TGRX-678 in patients with BCR::ABL1-positive CML and ALL.
- Patient Population: Included patients with CML or ALL harbouring the BCR::ABL1 fusion protein, including those resistant or intolerant to prior treatments.
- Endpoints:
- Primary Endpoints: Safety, dose-limiting toxicities (DLTs), and the MTD in Phase 1.
- Secondary Endpoints: Clinical responses, such as MMR, progression-free survival (PFS), and overall response rates (ORR).
Patient Characteristics in the TGRX-678 Study:
The TGRX-678 study included 158 patients across three arms during its dose expansion phase:
- Arm 1: CP patients with at least two prior TKI failures or were intolerant, and with no T315I mutation.
- Arm 2: CP patients with T315I mutations.
- Arm 3: Accelerated phase (AP) patients.
Key Demographics & Clinical Findings:
- Patient Composition: 1/3 were AP patients, and 2/3 were CP patients.
- Median Age: 46 years old.
- Time from CML Diagnosis to Starting TGRX-678: Median of 19.3 months.
- Mutation Insights:
- 27% of patients had only T315I mutations.
- 9% of patients had T315I mutations with other mutations.
- Nearly half of the patients had no mutations detected.
- Treatment History:
- Most patients had received or failed 3 to 4 prior TKIs.
- More than half of the patients had failed four or more lines of TKI therapies.
- 47 patients had previously failed treatment with, asciminib, olverembatinib, ponatinib, or other stem inhibitors.
- Treatment Duration & Discontinuation:
- Median treatment duration was 17 months.
- 32% of patients discontinued treatment due to disease progression.
- 8% discontinued treatment due to withdrawal.
- Only 4% discontinued due to adverse events.
- Current Treatment Status:
- 38% of patients remained on treatment as of the last data cutoff.
- Of these, 76% were in the CP group.
- More than half were in the AP group.
Results:
- CML-CP Patient Responses:
- 88% of patients achieved CHR.
- Nearly 30% achieved MCyR.
- Nearly half achieved CCyR, and 30% achieved MMR.
- CML-AP Patient Responses:
- 90% of patients achieved MaHR.
- 30-35% achieved MCyR and CCyR.
- >20% achieved MMR.
- Survival Metrics:
- In CP patients, PFS and OS reached 95% at 2 years.
- In AP patients, PFS and OS reached 80% at 2 years.
Safety and Tolerability
- Hematologic Toxicity: Thrombocytopenia and neutropenia were the most common severe AEs (Grade ≥3), occurring in nearly half of patients.
- Non-Hematologic Toxicity: Most frequent AE: Hyperlipidemia, generally mild and tolerable.
- Treatment Discontinuation: Only 5% of patients discontinued therapy due to AEs, underscoring the drug’s favourable tolerability.
- Dose interruptions/reductions: Reported in ~40–50% of patients.
Conclusion:
In conclusion, TGRX has demonstrated strong safety and efficacy profiles. No new safety signals were identified at the second year, confirming its continued safety. It is effective not only in CP patients but also in AP patients, including those with the T315I mutation who had previously failed therapy. While no strong correlation was observed with mutation topography, the results highlight TGRX's promising therapeutic potential across diverse patient subsets. Ongoing studies, such as the Phase 2 study in China and the Phase 1 study in the US, will further explore its utility and efficacy.
18-Months Follow-up of the Trial of Imatinib after Ponatinib Induction (TIPI) in the Front-Line Treatment of Chronic Phase-Chronic Myeloid Leukemia (CP-CML)
Speaker: Delphine Rea, French CML Group
Key Highlights:
Introduction:
Ponatinib is one of the most potent ATP-competitive inhibitors available, effective against both native and mutated BCR-ABL, as demonstrated in multiple clinical and model studies, including the PACE study. This led to the registration of ponatinib at 45 mg QD for patients resistant to second-generation or first- and second-generation TKIs, as well as those with resistance associated with the T315I mutation.
The OPTIC study optimized ponatinib dosing to balance efficacy with improved safety, particularly cardiovascular safety. Additionally, the EPIC trial explored ponatinib in the first-line setting compared to imatinib; however, this study was terminated early due to cardiovascular safety concerns despite promising early molecular responses.
Case Study:
- This case study highlights a patient, who was enrolled in the study and received ponatinib as a frontline therapy.
- The patient achieved an early and deep molecular response, reaching MR4 within just 3 months of treatment.
- After transitioning to imatinib at a dose of 400 mg daily, there was a temporary increase in residual disease during the switch; however, this was not sustained.
- Ultimately, the patient achieved a deep molecular response, allowing therapy to be discontinued after nearly 5 years.
- The patient has since maintained treatment-free remission (TFR) for an extended period.
This successful outcome from the case led to the design of the TIPI study to further investigate similar strategies.
Study Design: The study focused on optimizing ponatinib's use in patients to induce early deep molecular response while addressing cardiovascular safety.
- Main Inclusion Criteria:
- Age: Adults up to 65 years (to reduce cardiovascular risks linked to ponatinib).
- Diagnosis: Newly diagnosed EML patients only.
- Excluded patients with a low ELTS risk before the first safety analysis and significant cardiovascular history, except for controlled hypertension.
- The trial primarily enrolled intermediate- and high-ELTS-risk patients.
- Study Protocol:
- Ponatinib Administration: Started at 30 mg QD (lower than the standard 45 mg) for initial treatment.
- Transition to Imatinib: Patients transitioned to imatinib after 6 months.
- Objectives:
- Primary Objective: Measure the proportion of patients eligible for TFR, defined by the French CML group as:
- At least 3 years of treatment,
- At least 3 years of stable deep molecular response (MR4.0 or higher).
- Secondary Objectives:
- Assess efficacy, safety, PK data of ponatinib, and patient quality of life.
- Monitor compliance to both drugs (ponatinib and imatinib) during the study.
- Primary Objective: Measure the proportion of patients eligible for TFR, defined by the French CML group as:
Patient Characteristics: Total Enrolled Patients: 170 patients (169 treated)
- Median Age: 48 years
- Gender Distribution: More males than females.
- ELTS Risk: A higher proportion of intermediate and high ELTS-risk patients due to inclusion criteria.
- Cardiovascular Risk: Most participants had low to moderate cardiovascular risk, as per the European ESC score.
Results:
Efficacy Results
- Molecular Responses (Cumulative at 18 Months):
- MMR:
- 3 months: 14%
- 6 months: 57%
- 18 months: 72%
- MR4:
- 6 months: 30%
- 18 months: 45%
- MR4.5:
- 6 months: 11%
- 18 months: 21%
- MMR:
- Mutation Analysis:
- Mutations occurred primarily during imatinib maintenance, not ponatinib induction.
- Ponatinib's potent inhibition likely suppressed resistant clones.
- Few mutations were identified, underscoring the ability of ponatinib to prevent early resistance.
- Kinetics of Molecular Response: Rapid response during ponatinib induction (notably between 3–6 months) reflects its potency in clearing leukemic burden.
- Transition to imatinib led to a transient rebound in BCR::ABL1 levels, but responses stabilized over time.
Safety Profile:
- Ponatinib Induction Phase (6 Months):
- 81% experienced grade 3–5 AEs.
- Key AEs:
- Non-hematologic: Lipase elevation, liver enzyme increases.
- Cardiovascular: 4.5% (1 sudden death, hypertension, pulmonary embolism).
- Imatinib Maintenance Phase:
- AEs were less severe, with hypertension being the most common.
- Notably, no late cardiovascular events occurred following ponatinib discontinuation, suggesting no residual cardiovascular toxicity.
Conclusion:
The TIPI trial demonstrates that ponatinib induction followed by imatinib maintenance effectively achieves and sustains deep molecular responses in newly diagnosed CP-CML patients, particularly those at intermediate/high risk. This approach balances efficacy and safety, with ponatinib delivering early disease control and imatinib providing long-term maintenance with reduced toxicity.
Efficacy and Safety of Asciminib in Chronic Myeloid Leukemia in Chronic Phase (CML-CP): Interim Results from the Phase 2 ASC2ESCALATE Trial in the Cohort of Patients (Pts) after 1 Prior Tyrosine Kinase Inhibitor (TKI)
Speaker: Jorge Cortes, Georgia Cancer Centre, Augusta
Key Highlights:
Asciminib has demonstrated very high efficacy in patients with prior lines of therapy, including those who have been heavily pretreated. Currently, in the US, asciminib is approved for use across all lines of therapy, from frontline to post-therapy treatments.
The ASC2ESCALATE study is an interim analysis designed to evaluate the role of asciminib in both second-line therapy and first-line therapy, although the first-line data is not the primary focus of the analysis being presented. The study aims to further explore the efficacy of asciminib and its role in treatment strategies for patients across different therapy settings.
Study Design:
- Population: Chronic phase patients without the T359 mutation
- Cohorts:
- Second-line cohort (presented here)
- Frontline cohort
- Treatment at Enrolment: Standard dose of 80 mg asciminib once daily
- Evaluation at 6 Months: Patients with transcript levels >1% had the option to escalate to 200 mg once daily
- Evaluation at 12 Months: If no major molecular response was achieved, escalation options included:
- 200 mg once daily (for patients still at 80 mg)
- 200 mg twice daily (for patients already at 200 mg once daily)
- Primary Endpoint: Major molecular response at 12 months in the second-line cohort
- Total Patients Enrolled: 71
- Safety Analysis: Includes all patients who received at least one dose of aciminib
- Efficacy Analysis: Includes 28 patients who reached the first assessment for efficacy
- Efficacy Assessment Schedule: Weeks 4, 12, and 24
Patient Characteristics:
- Median Age: Under 50 years
- Race Distribution: Predominantly White and non-Hispanic patients
- Mutation Status: Only a few patients had mutations; none had the T359 mutation
- Prior TKI Usage:
- Dasatinib: Most common prior TKI, used by ~50% of patients
- Imatinib: Second most common TKI
- Nilotinib/Bosutinib: Fewer patients had received these therapies
- Duration of Prior TKI Use: Most patients had used their prior TKI for more than 1 year
- Median Duration of Exposure:
- Overall: 18 months (1.5 years)
- Median dose of asciminib: 80 mg daily
- 24-week Analysis:
- 85% of patients had continued therapy
- Median duration of exposure for these patients: 33 weeks
Results:
Efficacy:
- MMR: By Week 24 (approx. 6 months), >40% of patients achieved a MMR, highlighting promising efficacy.
- Response Subsets: Intolerant vs Resistant vs Lack of Efficacy:
- Efficacy was strong across all subsets.
- Patients with lack of tolerability and >10% initial transcripts showed the highest response rates, confirming drug efficacy rather than just a tolerability effect.
- Deep Molecular Responses:
- MR4 Response: Achieved by 25% of patients by week 24
- MR4.5 Response: Achieved by 10% of patients by week 24
Safety Profile:
- 88% of patients experienced any-grade AEs, with 25% experiencing grade ≥3 AEs.
- Most common toxicities:
- Nausea: 21%
- Vomiting: 17%
- Hypertension: 17% (11% grade 3)
- Thrombocytopenia was rare (5.6%).
Treatment Discontinuation and Adjustments:
- Only 1 patient discontinued therapy due to toxicity.
- Dose reductions occurred in 5 patients (7%).
- 13 patients required temporary treatment interruptions, but most resumed therapy without dose adjustment.
Conclusion:
The interim analysis of the ASC2ESCALATE trial demonstrates that asciminib is a highly effective and well-tolerated second-line therapy for CML-CP patients after failure or intolerance to 1 prior TKI. Early and deep molecular responses were achieved with minimal need for dose escalation, while safety outcomes remained favourable. These findings support the use of asciminib as a key option in the second-line setting, offering both efficacy and tolerability benefits.
Olverembatinib as Second-Line (2L) Therapy in Patients (pts) with Chronic Phase-Chronic Myeloid Leukemia (CP-CML)
Speaker: Weiming Li
Key Highlights:
Patients with CML often face relatively poor outcomes during second-line treatments and beyond. Olverembatinib, a third-generation BCR-ABL1 TKI, has shown remarkable efficacy and a favorable safety profile in patients with CML who are resistant or intolerant to at least two prior TKIs or those harboring T315I mutations. This makes it a promising therapeutic option for addressing unmet needs in this challenging patient population.
Aim of the Study: Assess the efficacy and safety of Olverembatinib as a second-line treatment for CP-CML patients without the T315I mutation.
Study Design:
- Single-arm, multicenter, open-label Phase 2 study
- Planned enrollment: 50 adult CP-CML patients
- Patients resistant or intolerant to one prior TKI
- Exclusion: Patients with T315I mutation
- Adequate organ function required when necessary
- Treatment: Olverembatinib 40 mg every other day (Dose proofed and commercialized in China)
- Primary Endpoint: CCyR rate
- Secondary Endpoints: CHR, MMR, MCyR, PFS, OS
Patient Characteristics:
- 43 patients enrolled:
- Median age: 45 years.
- 70% were male.
- Median time from diagnosis to trial start: >1 year.
- First-Line TKI Failure:
- Imatinib: 12 patients (28%).
- Second-generation TKIs: 31 patients (72%), including nilotinib, dasatinib, and flumatinib.
- Mutation Status:
- No mutations: 32 patients.
- Mutations (non-T315I): 11 patients.
Results:
- Overall Results (n=33):
- CCyR: 74.1%
- MMR: 40.6%
- Subgroup Analyses: Prior TKI:
- Second-generation TKIs (n=24):
- CCyR: 80%
- MMR: 43.5%
- Imatinib (n=9):
- CCyR: 50%
- MMR: 33.3%
- Second-generation TKIs (n=24):
- Baseline BCR::ABL Levels: BCR::ABL1 <10%:
- CCyR: 100%
- MMR: 50%
- BCR::ABL1 ≥10%:
- CCyR: ~70%
- MMR: 37.5%
- Mutation Status: No mutations:
- CCyR: 65%
- MMR: 34.8%
- Mutations (excluding T315I):
- CCyR: 85.7%
- MMR: 55.6%
Safety Profile:
- Non-Hematologic AEs:
- Most common: skin hyperpigmentation and hyperuricemia.
- Majority were Grade 1-2.
- Hematologic AEs:
- Common: platelet count decrease and anemia.
- Grade 3-4 events were manageable with supportive care.
- Serious AEs: Six patients experienced treatment-related serious AEs.
Conclusion:
- This is the first report on Olverembatinib as a second-line TKI therapy for CML patients.
- Olverembatinib demonstrated remarkable efficacy in patients with CPCML:
- Without T315I mutations
- Resistant or intolerant to prior first-line TKI treatment
- Including patients who failed first-line second-generation TKIs
- Patients with BCR-ABL mutations other than T315I
- No new safety signals emerged compared to previous reports.
- Preliminary data suggest that Olverembatinib is a viable second-line treatment option for CPCML patients, particularly for those failing first-line second-generation TKI therapy.
ASH Annual Meeting and Exposition, 7-10 December 2024, San Diego, California