ASCO 2025: Pediatric Oncology
Vincristine and Topotecan versus Carboplatin, Etoposide, and Vincristine-based Chemotherapy for Ocular Salvage in Group D and Group E Intraocular Retinoblastoma: A Randomized, Comparative Trial
Speaker: Prashant Prabhakar
Introduction
India accounts for the highest burden of retinoblastoma globally, with most patients presenting with advanced Group D or E disease. Ocular salvage rates in these cases remain poor (40-50%) worldwide. While intra-arterial chemotherapy has significantly improved globe salvage in Western countries, systemic chemotherapy remains the standard of care in low and middle-income countries like India due to lack of expertise, cost constraints, and logistical challenges.
Rationale for Topotecan-Based Therapy
Topotecan was chosen as a therapeutic option because it is devoid of long-term toxicities often associated with carboplatin and etoposide. Previous studies, like one by Brandon et al., had shown promising ocular salvage rates (70%) in Group D patients when topotecan was used alternately with carboplatin, although causality was difficult to attribute. Smaller studies also demonstrated topotecan's effectiveness in relapsed retinoblastoma settings.
Study Objectives
The study aimed to compare:
- Treatment failure rates between the two study arms after six cycles of chemotherapy.
- Hematological and non-hematological toxicity.
- Rate of decrease in tumor dimensions at the end of six cycles.
- Enucleation-free survival and final globe salvage rates.
Treatment failure was defined as any need for non-protocol-based systemic or intra-arterial therapy, external beam radiation therapy, or enucleation from enrollment until the completion of six cycles.
Study Design and Method
- This was a randomized, open-label, comparative trial conducted at the All India Institute of Medical Sciences (AIIMS), a major center for retinoblastoma patients. Patients were enrolled for 18 months, with a total study duration of three years, ensuring at least one year of follow-up after chemotherapy completion.
- Topotecan dosing was based on age, following a study by Adrenaline et al., due to the unavailability of therapeutic drug monitoring.
- Granulocyte colony-stimulating factor (G-CSF) was administered to patients in both arms 24 hours after chemotherapy.
Eligibility Criteria
- Included: Patients less than 12 years old, newly diagnosed with unilateral or bilateral intraocular retinoblastoma, having at least one eye with Group D or E disease.
- Excluded: Patients with extraocular disease, those who received prior systemic or intra-arterial chemotherapy, and Group E patients with high-risk features.
Data Collection and Monitoring
- Baseline: Demographic and anthropometric details, ophthalmic evaluation (examination under anesthesia, B-scans, MRI of brain and orbits), and relevant blood investigations were collected.
- Follow-up: Examination under anesthesia was performed after every two cycles, and toxicity was monitored after each cycle.
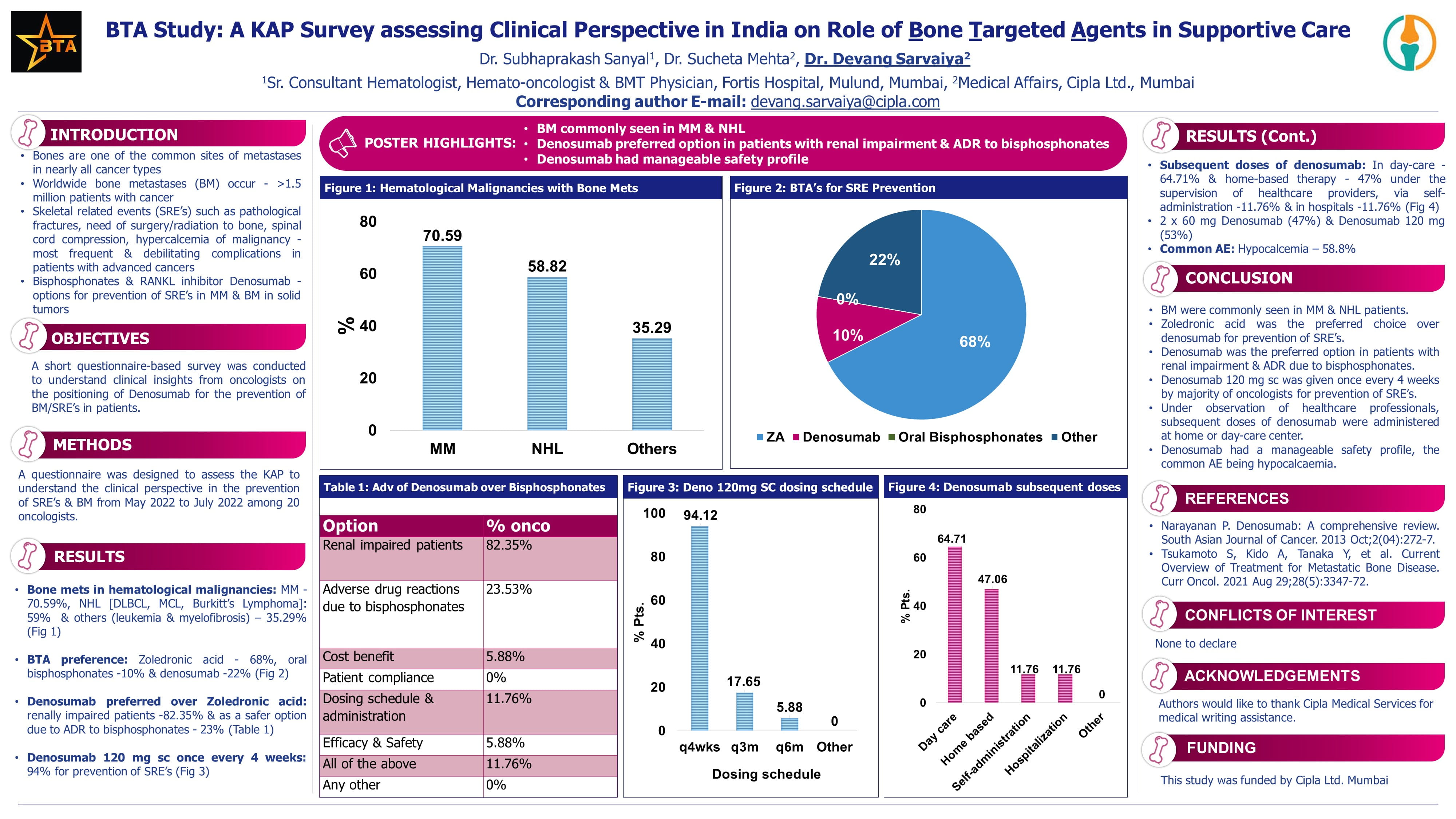
Results
Patient Enrollment and Allocation
- 47 patients were screened, and 41 patients were enrolled.
- 21 patients (22 eyes) were allocated to the carboplatin, etoposide, and vincristine (CEC) arm.
- 20 patients (21 eyes) were allocated to the vincristine and topotecan (VT) arm.
Treatment Outcomes and Failure Rates
- CEC Arm (22 eyes)
- 10 eyes experienced treatment failure (3 enucleations, 7 refractory disease requiring salvage therapy).
- 12 eyes were in remission at 6 cycles, with 3 reactivations during follow-up.
- Ultimately, 12 out of 22 eyes were salvaged at a median follow-up of 24 months.
- VT Arm (20 eyes)
- 15 eyes experienced treatment failure (50% enucleation, 50% refractory disease requiring salvage therapy).
- 5 eyes were in remission at 6 cycles, with 3 reactivations during follow-up.
- Ultimately, 7 out of 20 eyes were salvaged at the end of 24 months.
- Vision salvage was approximately 50% in both arms.
- An interesting finding in the VT arm was that 4 out of 5 patients who had refractory disease or reactivation achieved complete remission after only two cycles of carboplatin-based therapy.
Safety
- Topotecan had higher incidence of febrile neutropenia and diarrheal episodes.
- Carboplatin had more episodes of thrombocytopenia.
- No deaths or chemotherapy delays occurred in either arm due to toxicity.
Conclusion
Globe salvage remains poor in both Group D and E retinoblastoma patients. The topotecan-based regimen is inferior to the carboplatin-based regimen for globe salvage. Both regimens were well-tolerated, and topotecan administration is feasible without therapeutic drug monitoring. Initial tumor response was comparable for the first four cycles in both arms, but the effect was not sustained with topotecan.
Safety and Efficacy of the EZH1/2 inhibitor Valemetostat tosylate (DS-3201b) in Pediatric patients with Malignant Solid Tumors (NCCH1904): A Multicenter Phase I ELEPHANT Trial
Speaker: Ayumu Arakawa
Introduction
Valemetostat is a first-in-class EZH1/2 inhibitor that targets H3K27 methyltransferases EZH1 and EZH2. These enzymes are catalytic subunits of the PRC2 complex, which plays a crucial role in gene regulation. Valemetostat is expected to show anti-tumor activity against tumors harboring Swi/SNF mutations, such as SMARCB1/INI1 deficiency, often seen in childhood and adolescent cancers like ATRT and epithelioid sarcomas. Preclinical models also suggest that EZH2 inhibition combined with anti-GD2 immunotherapy could be a promising option for neuroblastoma.
Study Design
The NCCH1904 (ELEPHANT) trial is an open-label, multicenter Phase I trial.
Inclusion Criteria:
- Patients with relapsed, refractory, or progressive metastatic disease.
- In the dose escalation cohort, patients aged 3 to 19 years were enrolled.
- In the expansion cohort, patients aged 3 to 29 years were eligible.
- Patients who satisfied either or both of the following along with adequate organ function:
- One or more CT / MRI confirmed malignant solid tumor lesions
- Tumor infiltration confirmed by bone marrow examination
Methodology
- Three dose levels were assessed using a 3+3 design to determine the RP2D.
- Valemetostat was administered orally once daily without interruption in a 28-day cycle.
- The pediatric dose was based on adult doses, with Level 3 (250 mg/body equivalent) being the starting point.
Endpoints
- Primary Endpoint: Incidence of DLT.
- Secondary Endpoints:
- Incidence of adverse events (AEs).
- PK (parameters.
- Efficacy parameters: Objective response rate (ORR), Progression-Free Survival (PFS), and ORR for tumors with SMARCB1/INI1 deficiency.
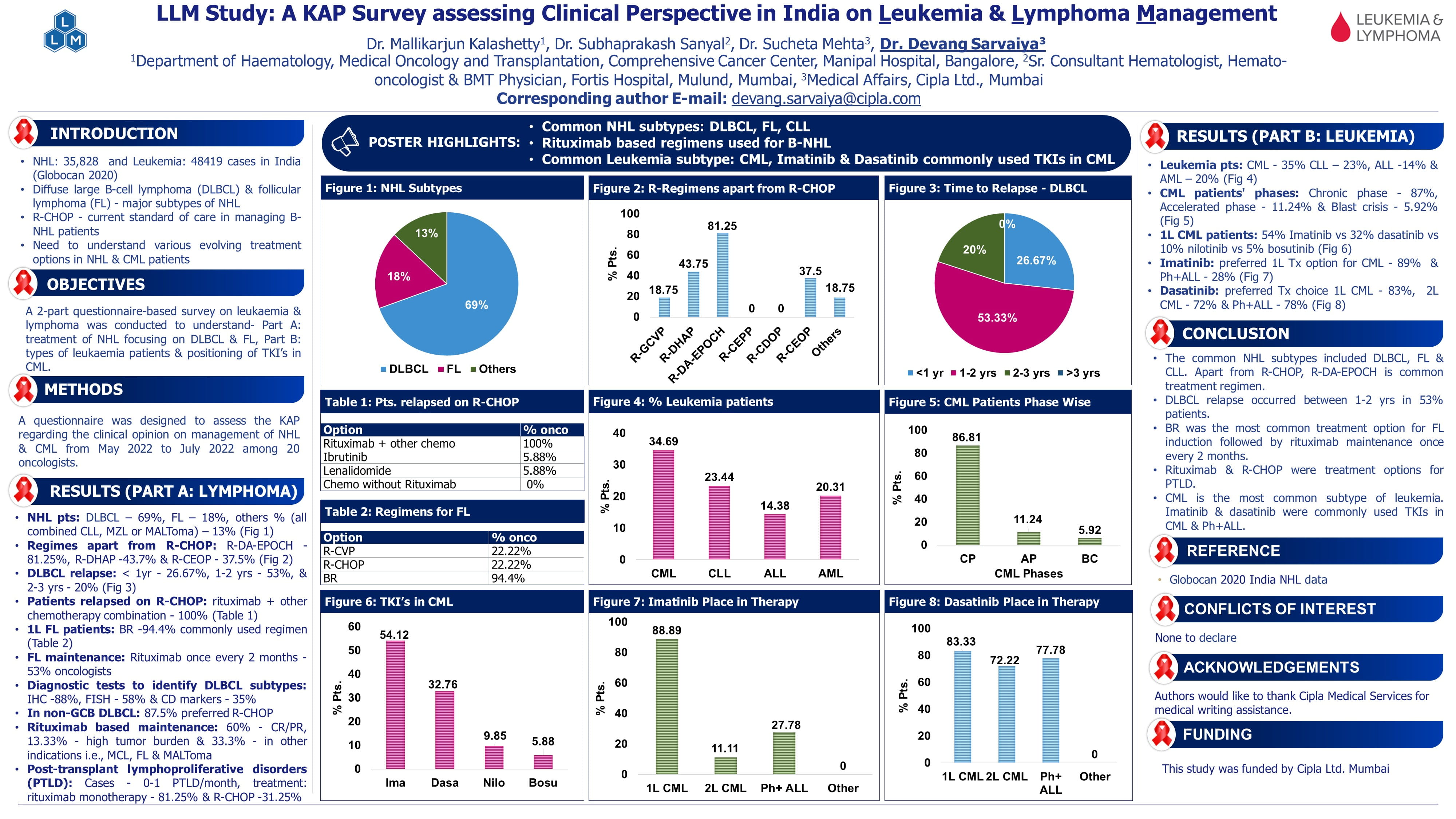
Patient Characteristics
- 30 pediatric and AYA patients were enrolled between March 2020 and January 2023.
- The median age was 8 years (3-19 years).
- Tumor types included:
- 8 neuroblastoma
- 6 ATRT
- 3 glioma
- 2 epithelioid sarcoma
- 1 extracranial MRT
- 43% of patients had INI1-negative tumors.
Results
Safety
- No DLTs were observed among 12 evaluable patients.
- The RP2D was established as 250 mg/body (Level 3 dose).
- 53% of patients experienced Grade >3 adverse events related to valemetostat, primarily hematological toxicities.
- One patient with neuroblastoma developed adjudicative myelodysplastic leukemia as a secondary malignancy, though it's likely linked to prior anti-cancer treatment.
- Notable treatment-related adverse events included Grade 3 PCP and pneumonitis (2 cases). These were managed with steroids and antimicrobial agents, allowing for treatment resumption. Prophylactic administration of trimethoprim-sulfamethoxazole is recommended.
Efficacy
- An objective response (PR) was observed in 2 out of 14 patients with measurable disease. Both were ATRT patients (2 out of 3 ATRT patients with measurable disease).
- No objective response was seen in patients with extracranial MRT, neuroblastoma, or glioma.
- Objective response was observed in 2 out of 6 patients with INI1-negative tumors, both being ATRT patients.
- Long-term disease control (exceeding one year) was noted in cases of neuroblastoma, chordoma, ATRT, and glioma.
- Specifically, three neuroblastoma patients showed disease control lasting over 18 months.
Case examples
- A 6-year-old girl with ATRT and multiple brain metastases achieved a partial response after 24 weeks of valemetostat, maintained for over a year.
- In one 19-year-old patient with epithelioid sarcoma and extensive metastases, some lesions reduced in size after 16 weeks, though a new intracranial lesion led to overall progressive disease.
- In neuroblastoma patients, where most had bone or bone marrow infiltration, disappearance of synaptophysin-positive neuroblastoma cells was observed in two patients, indicating potential anti-tumor effect.
Conclusion
Valemetostat can be safely administered to Japanese children at an RP2D of 250 mg/1.7 m2, with comparable PK to adults. Myelosuppression and infection (PCP/pneumonitis risk) are key adverse events requiring prophylactic trimethoprim-sulfamethoxazole. Moderate anti-tumor effects were observed in ATRT and epithelioid sarcoma, but the effect of a single agent might be limited.
Alectinib in Children and Adolescents with Solid or CNS tumors Harboring ALK-fusions: Data Update from the iMATRIX Alectinib Phase I/II Open-Label, Multi-center Study
Speaker: Francois Doz
Introduction
Alectinib has demonstrated anti-tumor activity in ALK-dependent preclinical models. Clinically, it is indicated and validated in ALCL (ALK-positive) and non-small cell lung cancer. ALK fusions, although rare, occur in various pediatric cancers, including ALCL, IMT (inflammatory myofibroblastic tumor), and infantile high-grade glioma.
Study Design
This is a phase I/II study evaluating the safety, pharmacokinetics, and efficacy of alectinib in children and adolescents with ALK fusion-positive solid or CNS tumors. The study includes patients for whom prior treatment has been ineffective or for whom no satisfactory treatment is available. The study is open in 12 countries. The reported data focuses on safety and efficacy, and dosage confirmation.
- Part 1: Dose Confirmation
- 22 patients were included.
Primary Endpoints
- Safety (incidence of dose-limiting toxicities during the first cycle).
- Pharmacokinetics (plasma concentration of alectinib and its metabolite).
- Efficacy (overall response rate).
Patient Characteristics
- Median age: 8 years (one-third of patients were below 2 years old).
- Median weight: 23.6 kg (lowest weight: 6 kg).
- Most patients had undergone surgery. Some had received chemotherapy and/or radiotherapy, often as first-line treatment.
- The ALK fusion partner varied.
- Tumor types were mainly primary, but some patients had metastatic tumors.
- Youngest children primarily had high-grade glioma (infantile type) or pleomorphic astrocytoma.
- Older children mainly had IMT. A variety of tumors with ALK fusions were observed across different ages.
Safety Profile
- The safety profile is consistent with that observed in adults.
- Five serious adverse events (SAE) were reported, two related to the drug.
- One dose-limiting toxicity: ALT increase above 12 times the normal value.
- Seven Grade ≥3 AE, five of which were drug-related.
- Related SAE: optic neuropathy, encephalopathy, and increased ALT.
Efficacy
- Prolonged treatment with alectinib was possible.
- A high proportion of partial responses were observed, including in high-grade glioma and pleomorphic astrocytoma.
- Response rates in IMT patients appear high.
- Alectinib was generally well-tolerated in pediatric patients with ALK fusion-positive solid or CNS tumors.
No related deaths or new safety signals were detected.
- Five treatment discontinuations: two due to progressive disease, two due to surgery, and one due to AE.
Recommended doses were determined for each weight category:
- Above 40 kg: 600 mg BID (same as adults).
- 20-40 kg: Using 300 mg capsules.
- Below 20 kg: 138 mg using a suspension.
Efficacy results are promising in this hard-to-treat population, with a majority of patients experiencing a response.
- Overall Response Rate (Intent-to-Treat Population): 72.7%.
- Overall Response Rate (Efficacy Evaluable Patients): 87.5%.
Conclusion
Alectinib was generally well tolerated in pediatric patients with ALK fusion-positive solid or CNS tumors. One dose-limiting toxicity (ALT increase) was observed, but no related deaths or new safety signals were detected. Recommended doses were determined for various weight categories, with patients above 40 kg receiving the adult dosage (600 mg BID). Efficacy results are promising in this hard-to-treat population, with a majority of patients experiencing a response, demonstrating a favorable benefit-risk profile. The response rate was 72.7% in the intent-to-treat population, and 87.5% in efficacy-evaluable patients.
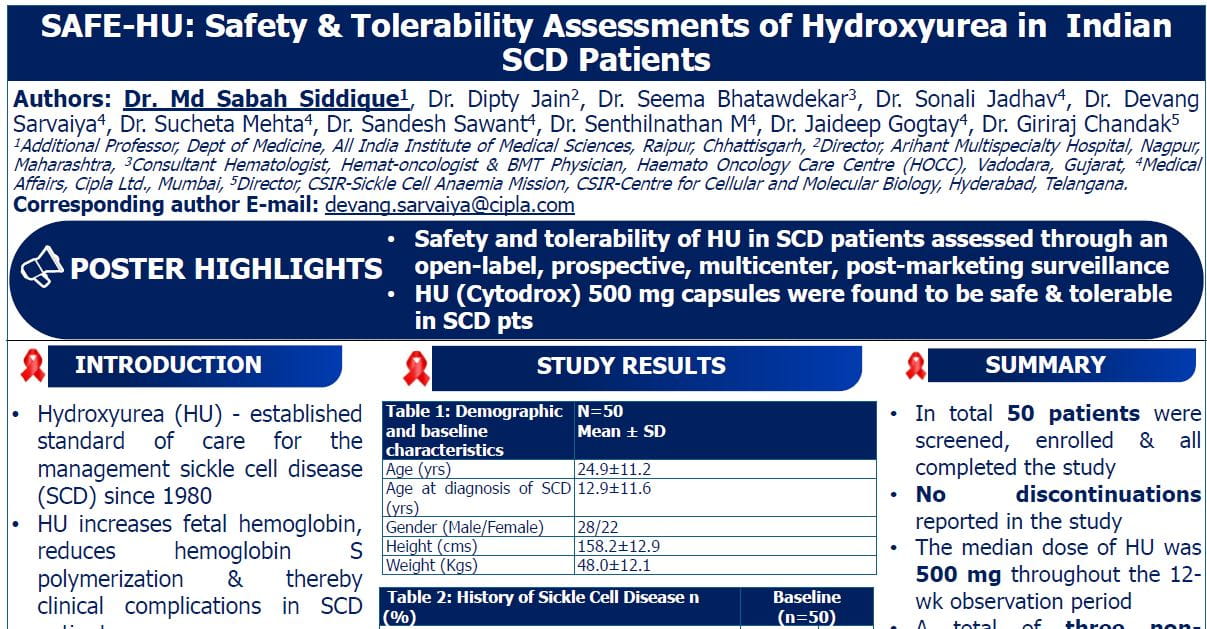
Results of Phase II Trial of Olaparib in Combination with Ceralasertib in Patients with Recurrent and Unresectable Osteosarcoma
Speaker: Suzanne Forrest
Introduction
- Olaparib is an oral selective PARP inhibitor, and ceralasertib is an oral selective inhibitor of ATR kinase.
- At the study's inception, several adult trials were already exploring this combination in other cancers like breast and ovarian cancers.
- The preclinical rationale for this trial stemmed from the in vitro susceptibility of osteosarcoma cell lines to ATR and PARP inhibitors. Additionally, approximately 20% of osteosarcoma cases exhibit loss-of-function variants in BRCA1, BRCA2, or ATR, indicating a potential vulnerability to these targeted therapies.
Study Design
This was a Phase II, single-arm, open-label, investigator-initiated study. It included two cohorts:
- Cohort 1: Patients with measurable disease (the efficacy cohort, reported here).
- Cohort 2: Patients with resectable disease limited to the lungs (to be reported later).
Key Eligibility Criteria for Cohort 1
- Age 12 to 40 years.
- Weight > 40 kg (due to utilizing adult dosing).
- RECIST v 1.1 measurable disease with no standard of care available, including surgical resection.
- No prior treatment with PARP or ATR inhibitors.
- Limited prior lung radiation due to a known, though rare, risk of pneumonitis associated with olaparib.
- Adequate performance status and organ function.
Objectives
- Primary Objective: To determine if the combination improved the four-month event-free rate.
- Secondary Objectives: Objective response rate, event-free survival, overall survival, safety, and tolerability.
- Exploratory Objectives: Relationship between DNA damage response pathway alterations and objective response, and evaluation of ctDNA as a surrogate marker.
Trial Design and Dosing
- The target was to increase the four-month event-free rate above a historical benchmark of 10-12% from prior COG trials (requiring 11 out of 34 patients, or 32%, to be event-free to meet the efficacy endpoint).
- Treatment was given in 28-day cycles for up to two years.
- The dosing strategy was amended early in the trial, with the final regimen being:
- Olaparib: 150 mg BID on days 1-28.
- Ceralasertib: 80 mg BID on days 1-14 of a 28-day cycle.
Patient Characteristics (Cohort 1)
- Median age: 19 years, with patients across the eligible age spectrum.
- Male predominance.
- Most patients were heavily pretreated (median of four prior regimens).
- 68% had received prior multi-TKI treatment.
Results
- Stage 1: 2 out of 15 patients were event-free at four months, allowing the study to proceed to Stage 2.
- Stage 2: 3 out of 19 patients were event-free.
- Overall (both stages): 5 out of 34 patients (15%) were event-free at four months. This did not meet the predetermined efficacy threshold of 32%.
- Objective Response Rate: 5.3%, with two patients achieving partial responses.
- Stable Disease: Seven patients had stable disease at the end of cycle 2; three continued to have stable disease at the end of cycle 4.
- Most patients experienced rapid tumor growth in the first two cycles, but a subset showed disease control beyond four months.
Case Examples (Partial Responses)
- Patient 1: A 16-year-old with recurrent metastatic osteosarcoma (left humerus, forearm recurrence). Heavily pretreated. Achieved a 59% tumor shrinkage (partial response) after two cycles, maintained for four and six cycles, but progressed after eight cycles.
- Patient 2: A 26-year-old male with metastatic osteosarcoma (femur, with extensive lung, liver, pericardial, and bone metastases). Also heavily pretreated. Achieved 47% tumor shrinkage (partial response) after two cycles, with significant reduction in lung nodules and liver burden.
- One patient with progressive disease after two cycles showed a notable metabolic response by PET scan and was later treated with olaparib and ceralasertib again via compassionate access for over a year.
Safety
The regimen was overall very well tolerated. The most common Grade 3+ treatment-related AEs were thrombocytopenia and anemia. The toxicity profile was similar to adult studies.
Conclusion
The study successfully demonstrated the feasibility of completing a single-site Phase II trial in recurrent osteosarcoma outside of a cooperative group setting in just two years. Most osteosarcoma patients in Phase II trials are heavily pretreated. While the primary efficacy endpoint was not met, the observation of objective partial responses, tumor shrinkage, and stable disease for over four months in some patients suggests a potential benefit from the ATR/PARP inhibitor combination.
ASCO 2025, May 30 – June 3, Chicago