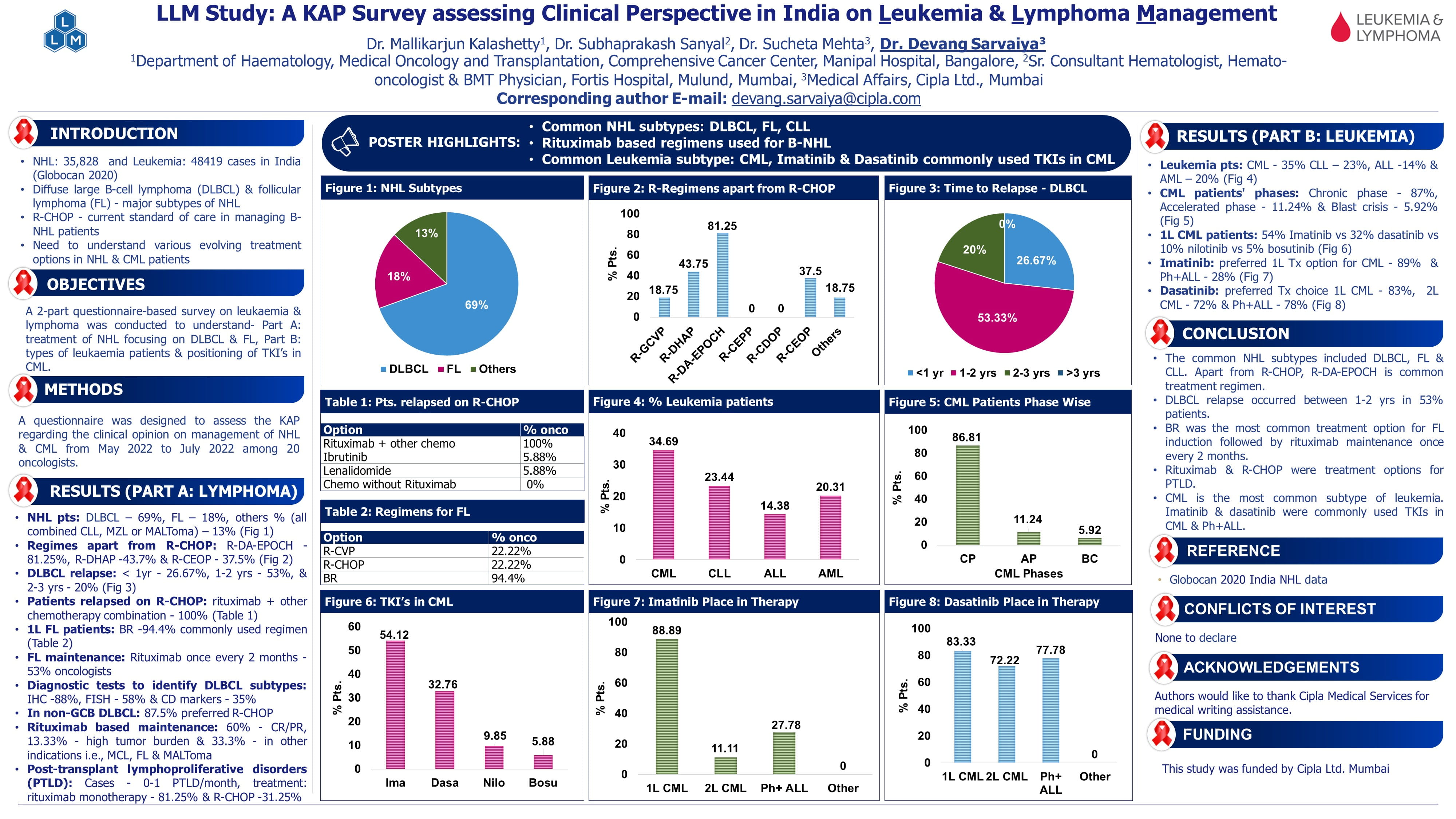
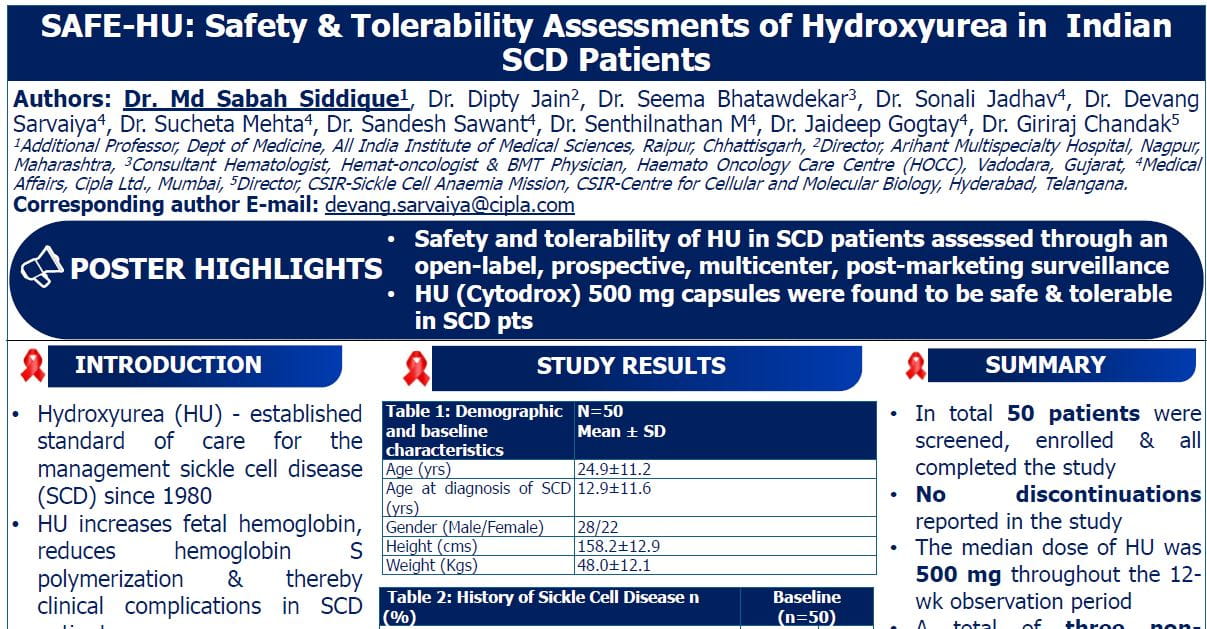
WaveLINE-003: Phase 2/3 Trial of Zilovertamab Vedotin Plus Standard of Care in Relapsed/Refractory Diffuse Large B-cell Lymphoma
Philippe Armand
Introduction
ROR-1 (Receptor Tyrosine kinase like Orphan Receptor-1) is a cell surface receptor re-expressed in many cancers, including relapsed/refractory diffuse large-cell B-lymphoma (R/R DLBCL), but not in normal adult tissues. Zilovertamab vedotin (ZV) is a novel antibody-drug conjugate targeting ROR1, carrying an MMAE microtubule toxin payload. Early phase studies of ZV monotherapy in R/R DLBCL showed a response rate of 29%. Rituximab, gemcitabine, and oxaliplatin (R-GemOx) is a standard second-line chemotherapy, especially for transplant-ineligible patients.
Study Objective
- Evaluate safety, tolerability, and preliminary efficacy of ZV + R-GemOx
- Determine the recommended phase 2 and 3 dose (RP2D) of ZV.
Methods
- Study Design
- Phase 2/3, open-label, multicenter, dose confirmation trial (WAVELINE-003) in R/R DLBCL after ≥1 lines of therapy (LOT) who were ineligible for chimeric antigen receptor T-cell therapy (CAR-T), autologous stem-cell transplant (ASCT), or failed such therapies.
- Three dose cohorts of ZV: 1.5, 1.75 (intermediate), and 2.0 mg/kg.
- Administered with fixed-dose R-GemOx every 3 weeks for up to 6 cycles.
- Inclusion Criteria
- Age and performance status allowing for outpatient chemotherapy.
- Confirmed R/R DLBCL, including:
- High-grade B-cell lymphoma.
- Double-hit lymphoma.
- ≥1 prior line of therapy.
- Transplant-ineligible or relapsed/refractory post-transplant.
- Exclusion Criteria
- Primary mediastinal B-cell lymphoma.
- Significant comorbidities that would preclude chemotherapy.
- Endpoints
- Primary: Safety, dose limiting toxicities (DLTs), and RP2D determination.
- Secondary:
- Objective response rate (ORR) and complete response (CR) by independent central review (Lugano criteria)
- Duration of response (DoR)
- Overall survival (OS)
Baseline Characteristics
- Total participants: 40
- 1.5 mg/kg: 17
- 1.75 mg/kg: 16
- 2.0 mg/kg: 7
- Median prior lines of therapy: 2 overall; 3 in high-dose cohort.
- Range: 1–7 prior therapies.
- Only 2 patients had prior exposure to polatuzumab vedotin (same payload as ZV).
Results
Safety
|
Measure |
1.5 mg/kg (n=17) |
1.75 mg/kg (n=16) |
2.0 mg/kg (n=7) |
|
DLTs |
1 |
2 |
4 |
|
Serious AEs |
24% |
— |
57% |
|
Grade 3–4 AEs |
53% |
— |
86% |
|
Treatment-related deaths |
0 |
0 |
1 (sepsis) |
|
Drug discontinuations |
0 |
0 |
2 |
- Common DLTs: Febrile neutropenia, ALT elevation, diarrhea, cytopenias.
- Peripheral neuropathy (expected due to ZV + oxaliplatin): low and no ≥ grade 3 events.
- Mandatory G-CSF added mid-study after protocol amendment.
Efficacy
|
ZV Dose |
ORR (%) |
CR (%) |
Median DoR |
Median OS |
|
1.5 mg/kg (n=15) |
27 |
20 |
14 months |
~12 mo |
|
1.75 mg/kg (n=16) |
56 |
50 |
9 months |
Not reached |
|
2.0 mg/kg (n=7) |
57 |
43 |
Not reached |
7 months |
- Median follow-up: ~10 months (longer for low-dose group).
- Higher response rates seen with 1.75 mg/kg, supporting its selection as RP2D.
Conclusion
Zilovertamab vedotin 1.75 mg/kg + R-GemOx demonstrated
- Promising efficacy (ORR 56%, CR 50%)
- Manageable safety profile
- Improved activity over historical R-GemOx alone
- Selected as the recommended Phase 2 and Phase 3 dose (RP2D)
- Study enrolling Phase 3 portion randomizing patients to receive ZV ± R-GemOx.
ASCO 2025, May 30 – June 3, Chicago