ASCO 2025: Hematologic Malignancies Highlights
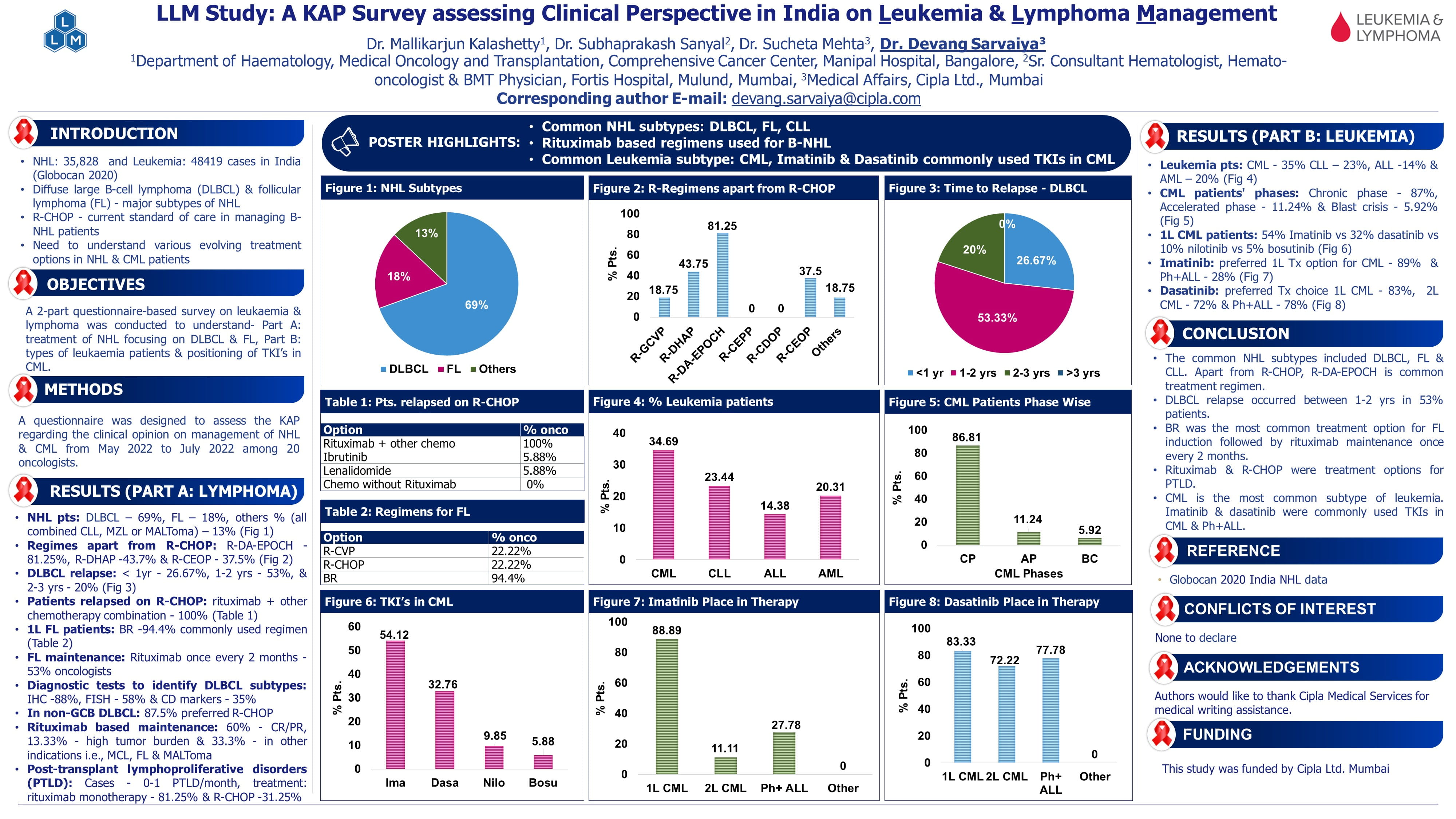
WaveLINE-003: Phase 2/3 Trial of Zilovertamab Vedotin Plus Standard of Care in Relapsed/Refractory Diffuse Large B-cell Lymphoma
Speaker: Philippe Armand
Introduction
ROR-1 (Receptor Tyrosine kinase like Orphan Receptor-1) is a cell surface receptor re-expressed in many cancers, including relapsed/refractory diffuse large-cell B-lymphoma (R/R DLBCL), but not in normal adult tissues. Zilovertamab vedotin (ZV) is a novel antibody-drug conjugate targeting ROR1, carrying an MMAE microtubule toxin payload. Early phase studies of ZV monotherapy in R/R DLBCL showed a response rate of 29%. Rituximab, gemcitabine, and oxaliplatin (R-GemOx) is a standard second-line chemotherapy, especially for transplant-ineligible patients.
Study Objective
- Evaluate safety, tolerability, and preliminary efficacy of ZV + R-GemOx
- Determine the recommended phase 2 and 3 dose (RP2D) of ZV.
Methods
Study Design
- Phase 2/3, open-label, multicenter, dose confirmation trial (WAVELINE-003) in R/R DLBCL after ≥1 lines of therapy (LOT) who were ineligible for chimeric antigen receptor T-cell therapy (CAR-T), autologous stem-cell transplant (ASCT), or failed such therapies.
- Three dose cohorts of ZV: 1.5, 1.75 (intermediate), and 2.0 mg/kg.
- Administered with fixed-dose R-GemOx every 3 weeks for up to 6 cycles.
Inclusion Criteria
- Age and performance status allowing for outpatient chemotherapy.
- Confirmed R/R DLBCL, including:
- High-grade B-cell lymphoma.
- Double-hit lymphoma.
- ≥1 prior line of therapy.
- Transplant-ineligible or relapsed/refractory post-transplant.
- Exclusion Criteria
- Primary mediastinal B-cell lymphoma.
- Significant comorbidities that would preclude chemotherapy.
- Endpoints
- Primary: Safety, dose limiting toxicities (DLTs), and RP2D determination.
- Secondary:
- Objective response rate (ORR) and complete response (CR) by independent central review (Lugano criteria)
- Duration of response (DoR)
- Overall survival (OS)
Baseline Characteristics
- Total participants: 40
- 5 mg/kg: 17
- 75 mg/kg: 16
- 0 mg/kg: 7
- Median prior lines of therapy: 2 overall; 3 in high-dose cohort.
- Range: 1–7 prior therapies.
- Only 2 patients had prior exposure to polatuzumab vedotin (same payload as ZV).
Results
Safety
|
Measure |
1.5 mg/kg (n=17) |
1.75 mg/kg (n=16) |
2.0 mg/kg (n=7) |
|
DLTs |
1 |
2 |
4 |
|
Serious AEs |
24% |
— |
57% |
|
Grade 3–4 AEs |
53% |
— |
86% |
|
Treatment-related deaths |
0 |
0 |
1 (sepsis) |
|
Drug discontinuations |
0 |
0 |
2 |
- Common DLTs: Febrile neutropenia, ALT elevation, diarrhea, cytopenias.
- Peripheral neuropathy (expected due to ZV + oxaliplatin): low and no ≥ grade 3 events.
- Mandatory G-CSF added mid-study after protocol amendment.
Efficacy
|
ZV Dose |
ORR (%) |
CR (%) |
Median DoR |
Median OS |
|
1.5 mg/kg (n=15) |
27 |
20 |
14 months |
~12 mo |
|
1.75 mg/kg (n=16) |
56 |
50 |
9 months |
Not reached |
|
2.0 mg/kg (n=7) |
57 |
43 |
Not reached |
7 months |
- Median follow-up: ~10 months (longer for low-dose group).
- Higher response rates seen with 1.75 mg/kg, supporting its selection as RP2D.
Conclusion
Zilovertamab vedotin 1.75 mg/kg + R-GemOx demonstrated
- Promising efficacy (ORR 56%, CR 50%)
- Manageable safety profile
- Improved activity over historical R-GemOx alone
- Selected as the recommended Phase 2 and Phase 3 dose (RP2D)
- Study enrolling Phase 3 portion randomizing patients to receive ZV ± R-GemOx.
Glofitamab plus Gemcitabine and Oxaliplatin (Glofit-GemOx) in Patients (pts) with Relapsed/Refractory (R/R) Diffuse Large B-cell Lymphoma (DLBCL): 2-year (yr) Follow-up of STARGLO
Speaker: Jeremy Abramson
Introduction
Glofitamab is a bispecific monoclonal antibody that targets CD20 on B-cells and CD3 on T-cells, redirecting T-cell cytotoxicity against malignant B-cells. It was initially approved as monotherapy for relapsed/refractory (r/r) DLBCL in the third-line or later setting, based on durable complete remission rates.
Study Design and Methods
STARGLO is a global, randomized, open-label, Phase 3 trial designed to evaluate the addition of (Glofit-GemOx) in patients with r/r DLBCL.
Patient Eligibility
- Adults with r/r DLBCL, not otherwise specified (NOS).
- Must have received ≥1 prior line of systemic therapy.
- Those with only one prior line had to be transplant-ineligible.
Randomization and Treatment Arms
- Total patients enrolled: 274
- Randomization: 2:1 ratio
- Glofit-GemOx
- R-GemOx, control arm
Treatment Regimen
- Both groups received 8 cycles (every 21 days) of GemOx-based chemoimmunotherapy.
- The Glofit-GemOx arm received:
- Step-up dosing of Glofit to mitigate CRS risk.
- Followed by 4 additional cycles of Glofit monotherapy (total 12 cycles).
- This regimen mirrors the dosing approach used in the Glofit monotherapy trials.
Endpoints
- Primary Endpoint: Overall Survival (OS)
- Key Secondary Endpoints:
- Progression-Free Survival (PFS)
- Complete Response (CR) Rate
- Safety profile
- Immune recovery (B-cell and Ig levels)
Baseline Characteristics
- Median age: 68 years
- 63% of patients had received only one prior line of therapy.
- 60% were refractory to their immediate prior therapy.
- Over 50% had primary refractory disease.
- Baseline characteristics were well balanced between arms.
Efficacy Outcomes
Overall Survival (OS)
- 40% reduction in risk of death with Glofit-GemOx.
- Median OS:
- Not reached in the Glofit-GemOx arm
- 5 months in the R-GemOx arm
- 2-year OS rate:
- 4% (Glofit-GemOx)
- 6% (R-GemOx)
Progression-Free Survival (PFS)
- Median PFS:
- 8 months (Glofit-GemOx)
- 6 months (R-GemOx)
- 59% reduction in risk of progression or death.
- At 18 months, twice as many patients on Glofit-GemOx were progression-free compared to R-GemOx.
Response Rates
- CR Rate:
- 5% (Glofit-GemOx)
- 25% (R-GemOx)
- Durability of Response: Among Glofit-GemOx patients who achieved CR:
- 89% were alive 1-year post-treatment.
- 82% remained progression-free.
Immune Recovery
- B-cell and immunoglobulin recovery began between 12–18 months.
- Full recovery observed in most patients by 18–24 months post-therapy.
- Recovery patterns were similar between the two arms.
Safety Profile
- No new safety signals emerged in the extended follow-up.
- Cytokine Release Syndrome (CRS):
- Occurred in 44.8% of patients on Glofit-GemOx.
- Mostly low-grade, limited to cycle 1, during step-up dosing.
- No Grade 5 adverse events or new late toxicities observed.
Conclusion
- Glofitamab plus GemOx continues to demonstrate sustained and clinically meaningful benefits in transplant-ineligible patients with r/r DLBCL.
- Durable OS, PFS and CR rates maintained at 2-year follow-up.
- Immune recovery occurred in most patients by 24 months.
- The combination offers a fixed-duration, off-the-shelf alternative with a manageable safety profile and supports its use in second-line and beyond, especially in transplant-ineligible patients.
Real-world Outcomes of Axicabtagene Ciloleucel (axi-cel) for the Treatment of Relapsed/refractory (r/r) Secondary Central Nervous System Lymphoma (SCNSL)
Speaker: Narendranath Epperia
Introduction
Historically, clinical trials evaluating CAR T-cell therapies—such as Axi-cel—have excluded individuals with CNS involvement due to concerns over neurotoxicity and uncertain efficacy. However, emerging real-world data suggest that CAR T-cell therapy may offer a viable treatment option for this high-risk population, with safety profiles similar to those seen in systemic cases.
This study aimed to assess the real-world effectiveness and safety of Axi-cel in patients with r/r sCNSL, providing valuable long-term follow-up data from routine clinical practice.
Study Design & Methods
- Type: Retrospective real-world study using CIBMTR registry data.
- Population: 65 adult patients with relapsed/refractory sCNSL receiving Axi-cel (2L+ setting) in the US (2018–2023).
- Follow-up Duration: Median 48.2 months.
- Key Subset: 56 patients without epidural involvement.
Patient Characteristics
- Median Age: 63 years.
- Performance Status: 84% had ECOG 0–1.
- Co-morbidities: Present in 80% of patients.
- CNS Involvement: Brain parenchyma was affected in 51%.
Efficacy Outcomes
- Objective Response Rate (ORR): 72%.
- Complete Response (CR): 51%.
- Median Duration of Response: 4 months.
- Response Durability: 35% at 2 and 3 years.
- Median PFS & OS: 3.6 and 8.4 months respectively.
- Findings consistent in patients without epidural involvement.
Safety Outcomes
- CRS (Grade ≥ 3): 14%.
- ICANS (Grade ≥ 3): 37%.
- Management: Corticosteroids and tocilizumab commonly used.
- Prolonged Cytopenias:
- Neutropenia: 11%.
- Thrombocytopenia: 37%.
- Infections: 60%.
- Secondary Malignancies: 3 cases (including leukemia, MDS).
- Death Rate: 63%.
- Safety profile consistent across subgroups.
Conclusion
- This is the longest real-world follow-up of CAR T-cell therapy in sCNSL.
- Axi-cel showed encouraging effectiveness and manageable safety profile, comparable to systemic cases.
- Additional studies are needed to enhance response durability and safety optimization.
- Supports Axi-cel as a potential treatment for relapsed/refractory secondary CNS lymphoma.
Efficacy and Safety of First-line Ibrutinib plus Venetoclax in Patients with Mantle Cell Lymphoma (MCL) who were Older or had TP53 Mutations in the SYMPATICO Study
Speaker: Michael Wang
Introduction
This presentation focuses on the results from the open-label, treatment-naive cohort of the SYMPATICO study, a Phase III trial evaluating the efficacy and safety of first-line ibrutinib plus venetoclax in patients with MCL, particularly those who are older or have TP53 mutations.
Addressing Unmet Needs in MCL
MCL is a challenging malignancy, especially in older patients who may not tolerate intensive chemotherapies. Furthermore, TP53 mutations, found in 15% to 20% of MCL patients, are associated with a high risk of disease progression and poor outcomes.
The combination of BTKi ibrutinib and BCL2i venetoclax leverages complementary modes of action and has shown synergistic effects in preclinical models of MCL. This synergistic activity forms the basis for investigating this combination in a clinical setting.
Study Design and Patient Characteristics
- This specific cohort of the SYMPATICO study enrolled 78 patients who received the combination therapy for two years, followed by single-agent ibrutinib. The median follow-up time on study was 40.5 months, and the median treatment duration was 24 months.
- The study included two main populations:
- Older Patients (≥65 years): This group comprised 83% of all enrolled patients. It included patients with and without TP53
- Younger Patients (<65 years) with TP53 Mutations: This group was specifically intended to enroll only TP53 mutated patients. However, only 11 patients were centrally confirmed to have TP53 mutations (two other patients were locally positive but centrally negative).
Key patient features and poor prognostic indicators at baseline included:
- Age: 83% patients ≥65 years.
- TP53 Mutation: 37%.
- High-Risk MIPI (MCL International Prognostic Index): 45% of patients.
- Blastoid Morphology: 31%.
- Extranodal Disease: 50%.
Results
Efficacy: The combination of ibrutinib and venetoclax demonstrated promising efficacy:
- ORR: 95%.
- CR Rate: 69%.
- Median Duration of CR and Duration of Response: 37.1 months.
- MRD Negativity: Achieved in the majority of patients with CR, both in bone marrow and peripheral blood.
- Median Time to Next Therapy: Not reached after 40.5 months of follow-up, indicating prolonged disease control.
- Median PFS: 42 months.
- Median OS: Not reached after 40.5 months of follow-up, indicating a very encouraging trend in OS.
Responses Across Subgroups
The combination notably improved response rates in patients with TP53 mutations:
- CR in Older Patients without TP53 Mutation: 77%
- CR in Older Patients with TP53 Mutation: 55%
While TP53 mutated patients generally had inferior PFS, the combination still allowed them to achieve a median PFS of 22 months, which is a significant outcome for this high-risk group.
Safety Profile
- The safety profile of the combination therapy was consistent with the known profiles of ibrutinib and venetoclax, with no new safety signals identified.
- Deaths: 7 patients (9%) died during the study, but none of these deaths were related to the study therapy.
- Most Frequent Toxicities: Diarrhea, fatigue, neutropenia, pneumonia, and COVID-19.
- Atrial Fibrillation: Occurred in 13 patients (17%). Grade 3/4 atrial fibrillation occurred in 4 patients (5%). No patient died from atrial fibrillation.
- Tumor Lysis Syndrome (TLS): No clinical TLS was observed, even with the early usage of venetoclax, suggesting effective prophylaxis and management strategies.
Conclusion
The SYMPATICO study's open-label cohort demonstrates that the first-line combination of ibrutinib plus venetoclax offers promising efficacy with high complete response rates and durable remissions in treatment-naive MCL patients, including those who are older or carry TP53 mutations. The safety profile is manageable and aligns with previous knowledge of these agents.
This combination may represent a valuable chemotherapy-free treatment option for older patients with MCL and for younger patients with TP53 mutations, addressing a critical unmet clinical need in this patient population.
ASCO 2025, May 30 – June 3, Chicago