ASCO 2025: Head and Neck Cancer Highlights
Patritumab Deruxtecan (HER3-DXd) in Active Brain Metastases (BM) from Metastatic Breast (mBC) and Non–small Cell Lung Cancers (aNSCLC), and Leptomeningeal Disease (LMD) from Advanced Solid Tumors: Results from the TUXEDO-3 Phase II Trial
Speaker: Dr. Mathias Preusser
Introduction
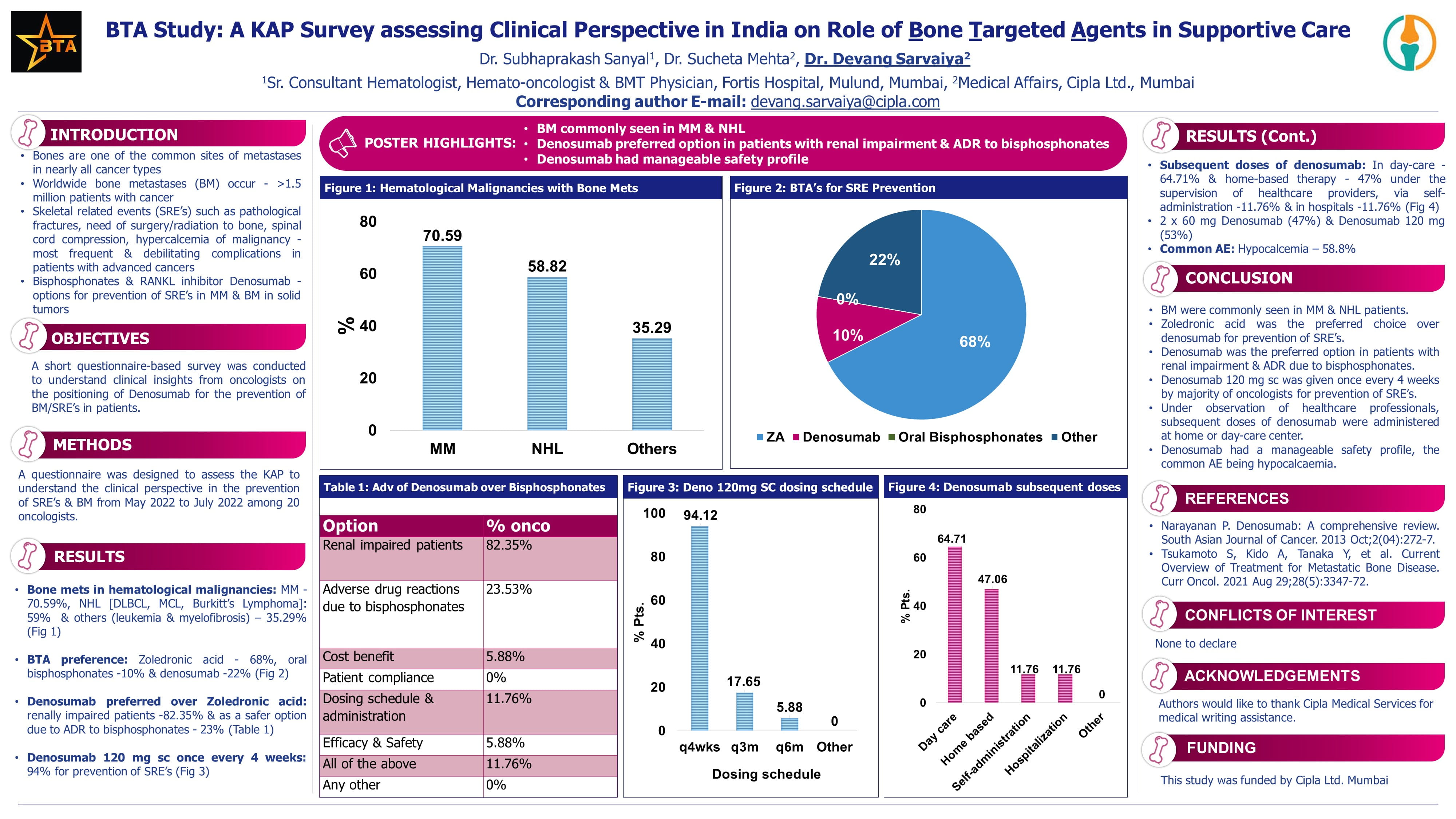
Brain metastases and LMD are common and serious complications of advanced cancers, especially breast and non-small cell lung cancer (NSCLC). HER3 is expressed in ~75% of brain metastases from breast and NSCLC. HER3-DXd is an antibody-drug conjugate (ADC) targeting HER3, linked to a topoisomerase I inhibitor. It has shown CNS penetration and potential efficacy. Previous trials investigated HER3-DXd in systemic settings (e.g., HERTHENA-Lung01); however, its CNS activity in active brain mets or LMD was unknown.
Study Design
- Trial Type: Phase 2, single-arm, open-label, non-comparative
- Three cohorts:
- Cohort 1: Breast cancer with active brain metastases
- Cohort 2: NSCLC with active brain metastases
- Cohort 3: LMD from any solid tumor
- Treatment: HER3-DXd at 5.6 mg/kg IV every 3 weeks
- Endpoints:
- Primary
- Cohorts 1 & 2: Intracranial ORR, by RANO-BM criteria
- Cohort 3: 3-month OS rate
- Secondary: Extracranial ORR, PFS, OS, safety, QoL, neurocognitive and neurological function
- Primary
Patient Characteristics
- Enrolled: 60 patients between Dec 2023–Sep 2024 across 8 sites (Austria & Spain)
- Median age: 50–60 years
- ~30% had treatment-naive with active brain metastases
- Cohort 3: All newly diagnosed, no prior LMD treatment
- Tumor Subtypes
- Cohort 1 (Breast cancer): HER2+, luminal, and triple-negative
- Cohort 2 (NSCLC): Squamous and non-squamous histology, EGFR WT and mutant
- Cohort 3 (LMD): Mostly breast or lung primary, 50% with CSF+ (type 1)
Results
Efficacy
- Cohort 1 – Breast Cancer with Brain Mets
- Intracranial ORR: 23.8%
- Responses across subtypes, including deep responses in triple-negative BC
- Activity noted even in patients with prior ADC exposure
- Cohort 2 – NSCLC with Brain Mets
- Intracranial ORR: 30%
- Majority of responders had EGFR WT tumors and non-squamous histology
- Effective in both newly diagnosed and previously treated brain metastases
- Cohort 3 – LMD from any Solid Tumors
- 3-month OS rate: 65%
- 10-month OS: ~60%
- No difference between LMD type 1 and 2
- Suggests meaningful activity and durable survival in this poor-prognosis population
Secondary Outcomes
- Intracranial responses > extracranial responses
- DcR and clinical benefit rate (≥6 months) were encouraging despite prior heavy treatment
Safety Profile
- Grade ≥3 treatment-emergent AEs: ~33%
- No HER3-DXd related deaths
- Permanent discontinuation in ~5% of patients
- Common AEs: Neutropenia, nausea, diarrhoea
Quality of Life & Neurocognition Function
- Assessed via standardized tools
- QoL and cognitive function remained stable or improved across treatment period
Conclusion
- HER3-DXd demonstrated CNS activity in: Brain metastases from breast and lung cancers, Leptomeningeal disease (LMD) with significant survival benefit
- Effective across subtypes, including:
- Triple-negative BC
- EGFR-WT NSCLC
- After prior ADC use
No new safety signals; well-tolerated with preserved QoL and function
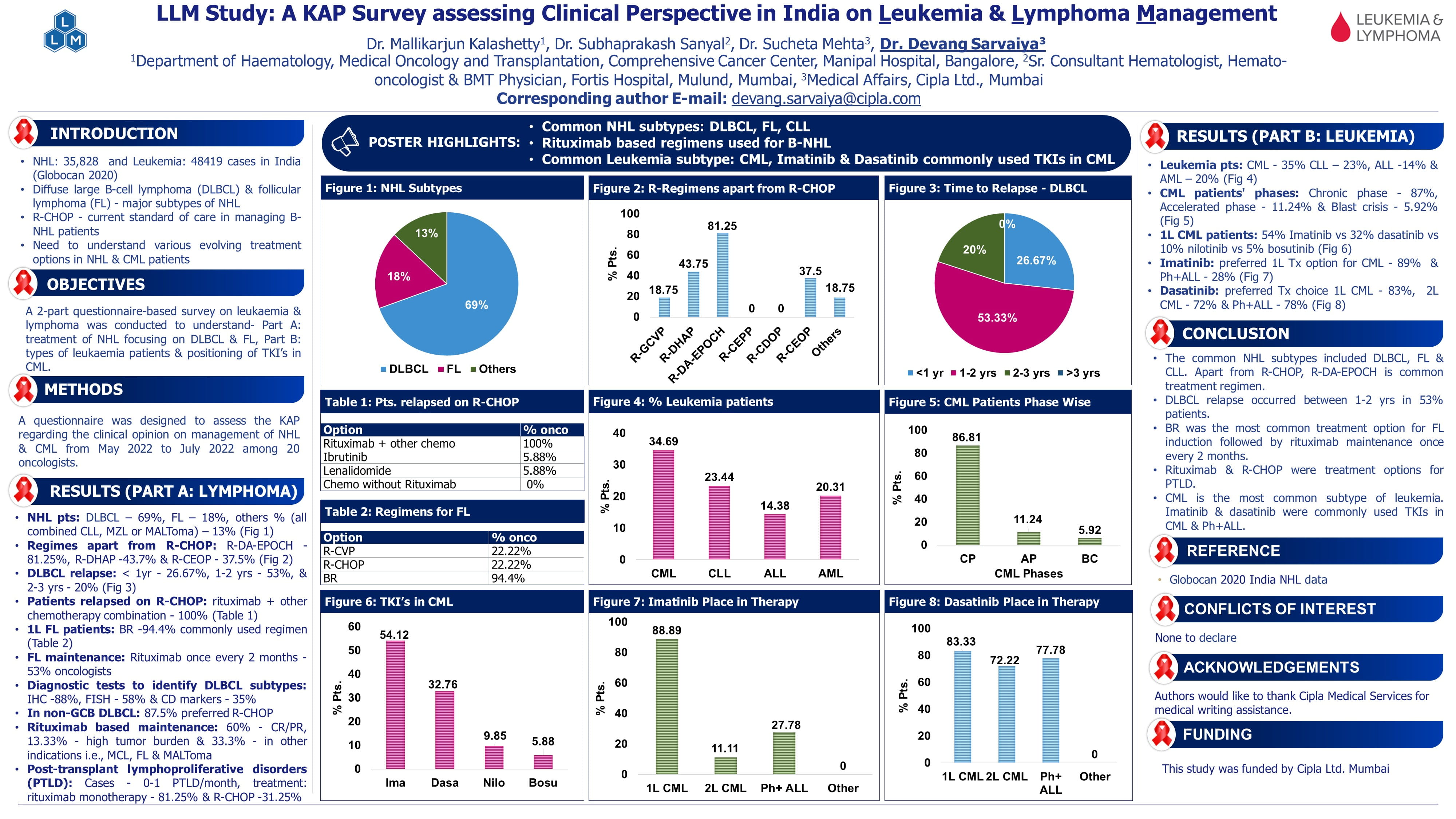
Long-term Results of the Randomized, Phase 3 KEYNOTE-412 Trial of Pembrolizumab (Pembro) or Placebo (PBO) plus Concurrent Chemoradiotherapy (CRT) for Unresected, Locally Advanced Head and Neck Squamous Cell Carcinoma (LA-HNSCC)
Speaker: Dr. Yungan Tao
Introduction
The KEYNOTE-412 trial was initiated to evaluate whether adding Pembro to concurrent CRT could improve outcomes in patients with unresected, LA HNSCC. In the initial analysis conducted three years ago, Pembro plus CRT did not significantly improve event-free survival (EFS), with a hazard ratio of 0.83 and 363 EFS events recorded out of the planned 410. This earlier analysis had a median follow-up of about 4 years.
Given these inconclusive early results, longer follow-up was deemed necessary to fully assess the potential clinical benefit. The updated presentation includes over 2 additional years of follow-up, allowing a more complete evaluation of Pembro’s effect on EFS, OS, and other key endpoints in this population.
Study Design: KEYNOTE-412 (NCT03040999)
Population: 804 patients with high-risk, unresected LA HNSCC were randomized 1:1 into two treatment arms.
Eligibility Criteria (High-risk unresected LA HNSCC):
- Oropharyngeal p16-positive
- T4 (N0–N3), M0
- (T1–T4) N3, M0
- Oropharyngeal p16-negative:
- Any T3–T4 (N0–N3), M0
- (T1–T4) Any N2a–3, M0
- Larynx, hypopharynx, oral cavity (p16-independent):
- Any T3–T4 (N0–3), M0
- (T1–T4) Any N2a–3, M0
Treatment Arms
- Arm A:
- Pembro (200 mg Q3W) + concurrent CRT
- Followed by 14 maintenance doses of pembro
- Arm B:
- Placebo + concurrent CRT
- Followed by 14 maintenance doses of PBO
- CRT Regimen: 70 Gy in 35 fractions + high-dose cisplatin (100 mg/m² Q3W) or placebo Q3W
Endpoints
- Primary: Event-Free Survival (EFS)
- Key Secondary:
- Overall Survival (OS)
- Exploratory:
- Locoregional control
- Distant metastasis-free survival (DMFS)
- Efficacy by PD-L1 expression: CPS ≥1 and CPS ≥20
Follow-up
- Median study follow-up: 74.4 mo (range: 63.7–88.1 mo)
- Patients were followed for disease evaluation, and in cases of progression, entered survival follow-up.
Key Results
|
Outcome |
Pembro + CRT |
PBO + CRT |
HR (95% CI) |
60-mo Rate (%) |
|
Event-Free Survival (ITT) |
71.8 mo (55.4–NR) |
49.8 mo (26.8–66.2) |
0.79 (0.65–0.96) |
54.7 vs 47.2 |
|
Overall Survival (ITT) |
NR (NR–NR) |
NR (74.3–NR) |
0.86 (0.70–1.07) |
64.4 vs 59.8 |
|
EFS (PD-L1 CPS ≥20) |
NR (71.8–NR) |
65.9 mo (34.3–NR) |
0.70 (0.49–1.00) |
64.4 vs 51.8 |
|
OS (PD-L1 CPS ≥20) |
NR (NR–NR) |
NR (NR–NR) |
0.73 (0.49–1.07) |
72.7 vs 62.5 |
|
Distant PD Events (%) |
56 (13.9%) |
72 (17.9%) |
— |
— |
|
Locoregional PD Events (%) |
54 (13.4%) |
60 (14.9%) |
— |
— |
|
Deaths (%) |
64 (15.9%) |
64 (15.9%) |
— |
— |
NR = Not Reached; CRT = Chemoradiotherapy; PD = Progressive Disease; EFS = Event-Free Survival; OS = Overall Survival; ITT = Intention-to-Treat
Conclusion
- Pembro + CRT demonstrated a clinically meaningful EFS improvement, with trends toward improved OS and DMFS.
- The benefit increased with PD-L1 expression, suggesting PD-L1 could serve as a predictive biomarker.
- Longer follow-up may be needed to confirm OS advantages.
- The combination may become a preferred strategy in selected patients with unresected LA HNSCC.
Phase 3 Randomized Trial (KEYNOTE-630) of Adjuvant Pembrolizumab (Pembro) versus Placebo (PBO) for High-risk Locally Advanced Cutaneous Squamous Cell Carcinoma (LA CSCC) following Surgery and Radiation Therapy (RT)
Speaker: Dr.Jenny Lee
Introduction
Dr. Jenny Lee presented findings from the KEYNOTE-630 phase 3 randomized, double-blind trial evaluating adjuvant Pembro vs PBO in patients with high-risk LA-CSCC following surgery and RT. SoC for LA-CSCC is surgery followed by postoperative RT. Up to 30% of patients experience recurrence within 5 years. PD-1 inhibitors like Pembroand cemiplimab are approved for unresectable or metastatic CSCC based on prior phase 2 efficacy. The study aimed to improve outcomes in a population with high recurrence risk post-standard care.
Study Design
- Type of Study: Randomized, double-blind, phase 3 trial.
- Sample Size: 450 participants were randomized.
- Treatment Arms:
- Arm 1: Pembrolizumab 400 mg every 6 weeks, up to 9 cycles
- Arm 2: Placebo (same schedule)
- Crossover Design:
- Placebo arm participants could cross over to pembrolizumab upon recurrence.
- Pembrolizumab arm participants could receive retreatment upon recurrence if ≥6 months had passed since completion.
- Stratification Factors:
- Extracapsular extension
- Cortical bone invasion
- Prior systemic therapy
- Endpoints:
- Primary: Recurrence-free survival (RFS)
- Secondary: Overall survival (OS), safety, tolerability
- Follow-Up Duration: Median follow-up was 28.6 months.
Eligibility Criteria
Participants had to meet all the following:
- Diagnosis: High-risk LA-CSCC
- Treatment history:
- Underwent curative-intent surgery
- Completed adjuvant RT within 4 to 16 weeks before randomization
- High-risk features (patients had to meet one of the following):
- Lymph node metastasis with:
- Extra-nodal extension, or
- ≥1 node >2 cm, or
- Multiple lymph nodes involved
- Primary tumor with ≥2 of the following:
- Tumor size ≥4 cm
- Depth >6 mm or invasion beyond subcutaneous fat
- Multifocal perineural invasion
- Involved node ≥10 mm
- Poor differentiation or sarcomatoid histology
- Recurrent disease within 3 years of local treatment
- Satellite or in-transit metastasis
- Lympho-vascular invasion
- Gross cortical bone invasion or skull base invasion
- Lymph node metastasis with:
Results
|
Endpoint |
Pembro |
PBO |
Significance |
|
24-month RFS |
78.3% |
68.6% |
HR: 0.76, p = 0.07243 (NS) |
|
24-month OS |
87.3% |
90.7% |
NS |
|
Cumulative recurrence |
31 cases (13.9%) |
57 cases (28.1%) |
Not statistically powered |
- Subgroup analysis showed age >65, non-smokers, and nodal metastasis with extracapsular extension favored pembrolizumab for RFS.
- No OS benefit observed.
- Higher deaths in the pembrolizumab group; many were non-disease related, possibly affected by COVID-19.
Crossovers and Retreatment
- 30 PBO patients crossed over to pembro post-recurrence; ~50% responded.
- Some pembro group patients received retreatment upon recurrence.
Safety
- Any-grade AEs: 63.8% (Pembro) vs. 41.1% (PBO)
- Grade 3–4 AEs: rare
- No treatment-related deaths
- Most common AEs: Pruritus, hypothyroidism, fatigue, rash, diarrhea, arthralgia
- Immune-related AEs were consistent with prior pembro trials.
Conclusion
Pembrolizumab did not achieve statistically significant RFS improvement over placebo. Lower recurrence and metastasis rates seen but offset by higher unexplained mortality. No new safety concerns; AE profile matched known data. Further research (e.g., HNO-14 trial) will explore neoadjuvant vs. adjuvant immunotherapy in this setting.
Phase 3 trial of adjuvant Cemiplimab (cemi) versus Placebo (PBO) for high-risk cutaneous Squamous cell Carcinoma (CSCC) [C-POST]
Speaker: Dr. Danny Rischin
Introduction
Dr. Danny Rischin presented findings from the C-POST Phase 3 trial, evaluating adjuvant cemiplimab in patients with high-risk CSCC who had undergone surgery and radiotherapy. The trial demonstrates the first statistically significant benefit of a systemic therapy in this setting.
Study Design
- Type: Global, multicenter, randomized, double-blind, placebo-controlled Phase 3 trial
- Randomization: 1:1 to cemiplimab or placebo
- Arms:
- Cemiplimab 350 mg IV Q3W for 48 weeks (early protocol)
- Updated to 700 mg IV Q6W after 12 weeks (post June 2021 amendment)
- Crossover: Placebo group patients who relapsed could cross over to cemiplimab in Part 2
- Stratification Factors: 5 total, including CLL status
- Primary Endpoint: Disease-Free Survival (DFS)
- Secondary Endpoints: Freedom from local/regional/distant recurrence, second primary CSCC, overall survival (OS), and safety
Inclusion Criteria
- Gross resection of all pathologically confirmed disease and ECOG 0–1
- Completed adjuvant RT (≥50 Gy BED) within 2–10 weeks before randomization
- Adequate hepatic, renal, and bone marrow function
- Must have ≥1 high-risk feature:
- Nodal: ≥3 nodes or ≥1 node with ECE and size ≥20 mm
- Non-nodal: T4 lesion, in-transit metastases, perineural invasion (named nerve involvement), recurrent CSCC + 1 other risk factor
Exclusion Criteria
- SCC of non-cutaneous origin
- History of solid organ transplant
- Recent or active malignancy (except untreated stable CLL with no treatment in past 6 months)
- Uncontrolled comorbidities
Patient Characteristics
|
Variable |
Cemiplimab Arm |
Placebo Arm |
|
Patients randomized |
209 |
206 |
|
Median age |
71 years |
Similar |
|
Gender |
Predominantly male in both arms |
|
|
Primary tumour site |
Mostly head and neck |
|
|
High-risk nodal disease |
58% |
Similar |
|
Most common risk factor |
Nodal ECE ≥20 mm |
48% overall |
Results
Efficacy Outcomes
- Primary Endpoint – DFS
- Events: 24 (cemi) vs. 65 (PBO)
- HR: 0.319, p-value: <0.0001
- 2-year DFS:
- Cemiplimab: 87%
- Placebo: 64%
- Median DFS not reached in cemiplimab arm; 49.4 mo in PBO arm
- Subgroup Analyses:
- DFS benefit consistent across all subgroups, including:
- Nodal and non-nodal high-risk
- PD-L1 positive and negative
- By Dosing Schedule
- Q3W group HR: 0.44
- Q6W switch group HR: 0.25
- DFS benefit consistent across all subgroups, including:
Secondary outcomes from the CPOST trial as bullet points
- Freedom from local/regional recurrence: HR: 0.20 which indicates strong reduction in locoregional relapse with cemiplimab
- Freedom from distant recurrence: HR: 0.35 which demonstrates reduced risk of metastatic spread
- OS:
- Primary analysis (25 deaths): HR 0.86
- Updated analysis (33 deaths, April 2025): HR 0.78
- 95% CI: 0.39–1.56 → not statistically significant
Safety Profile
|
Adverse Event Category |
Cemiplimab |
Placebo |
|
Any grade ≥3 AE |
24% |
14% |
|
AEs leading to treatment discontinuation |
10% |
1% |
|
Treatment-related deaths |
2 |
2 |
|
Grade ≥3 immune-related Aes |
7% |
NA |
|
Most common Aes |
Fatigue, pruritus, rash, diarrhea |
Conclusion
- Cemiplimab is the first systemic therapy to significantly reduce disease recurrence in high-risk CSCC in the adjuvant setting.
- Improved local, regional, and distant recurrence outcomes
- Acceptable and consistent safety profile with known use in advanced CSCC
- Cemiplimab represents a potential new standard of care for patients with high-risk CSCC post-surgery and RT
Rare But Real: New Therapeutic Options
Speaker: Dr. Alan L. Ho
Introduction
Clinical trials in rare cancers exemplify the progress of precision oncology. The three presented abstracts show how targeted therapies and trial design innovations have driven progress in ultra-rare tumors. Notable advances were seen in salivary gland cancers (AR and HER2 targeted therapies) and anaplastic thyroid cancer (triplet therapy with BRAF/MEK + immunotherapy).
AR-Positive Salivary Gland Cancers
Background
- Salivary duct carcinomas are aggressive, predominantly affecting older males.
- AR (androgen receptor) is the most common target, expressed in >70% of patients.
Therapeutic Developments
- Early Japanese trials using biclutamide + ADT showed 42% response and 9-month PFS.
- Apalutamide + ADT underperformed compared to first-gen antiandrogens.
- Enzalutamide alone had poor efficacy (4% response), emphasizing the need for ADT.
- Abiraterone (CYP17A1 inhibitor) showed activity in castration-resistant cases.
Darolutamide Phase 2 Data
- Best response seen so far in AR therapy-naive salivary cancers.
- Response rate and PFS superior to previous AR-targeted studies.
- Higher proportion of patients had >70% AR staining, potentially explaining better outcomes.
- HER2 overexpression might mediate resistance to AR therapy; under-evaluated in prior trials.
HER2-Positive Salivary Gland Cancers
Background
- HER2 overexpression occurs in ~1/3 of salivary carcinomas; HER2-low also recognized.
- HER2-targeting with docetaxel + trastuzumab achieves ~70% response rate.
Antibody-Drug Conjugates (ADCs)
- TDM1 and TDXD showed promising responses and longer PFS than trastuzumab-docetaxel.
- SHR (trastuzumab deruxtecan, referred here as Trastuzumab Resatikan) achieved:
- 81% ORR and 100% DCR in HER2-overexpressing cohort.
- 20% ORR in HER2-low cohort (2/10 patients responded).
- HER2-low cohort failed to meet statistical threshold for expansion, partly due to histological heterogeneity.
Limitations & Ongoing Trials
- Small sample sizes and varied histologies limit conclusions.
- Ongoing TDXD trials in HER2-low cancers may help clarify benefit (e.g., NRG HN-10, Mythos).
- SHR showed fewer discontinuations and lower pneumonitis risk than TDXD.
Treatment Sequencing Challenges
- With growing options (AR-targeted, HER2-targeted, ADCs), sequencing is unclear.
- NRG HN-10 is comparing trastuzumab-docetaxel vs. TDM1 to clarify first-line therapy.
Anaplastic Thyroid Cancer (ATC): Triplet Therapy Advances
Background
- ATC is highly aggressive with poor survival.
- Prior studies showed efficacy of dabrafenib-trametinib (DT) in BRAF-mutant ATC (ROAR study: 56% ORR, >1-year OS).
New Triplet Regimen: Dabrafenib-Trametinib-Pembrolizumab (DTP)
- Neoadjuvant DTP resulted in:
- 77% surgical conversion.
- 74% R0/R1 resection rate.
- 67% pathologic complete response.
- Shows added value of checkpoint inhibition, especially in immunogenic tumor microenvironment.
Conclusion
- Major therapeutic progress has been made for rare cancers.
- DTP in ATC and AR/HER2-targeted strategies in salivary cancers offer hope.
- But optimal treatment sequencing and validation via randomized or innovative designs (e.g., synthetic control arms) remain key next steps.
Tagitanlimab versus Placebo (PBO) in Combination with Gemcitabine and Cisplatin as First-line Treatment for Recurrent or Metastatic Nasopharyngeal Carcinoma (R/M NPC): Results from a Randomized, Double-blind, Phase 3 Study
Speaker: Dr. Haiqiang Mai
Introduction
NPC is prevalent in East Asian populations, with China bearing the highest disease burden. It's characterized by a highly immunogenic tumor microenvironment, making immunotherapy a promising treatment option. Currently, platinum-based chemotherapy combined with PD-1 inhibitors is the standard first-line treatment for R/M NPC.
Tagitanlimab is a humanized anti-PD-L1 monoclonal antibody with a unique PD-L1 antigen-binding epitope, excellent pharmaceutical properties, and a good safety profile. Previous trials showed promising efficacy in R/M NPC patients with fewer than two lines of chemotherapy.
Study Design
This phase 3 study included patients with histologically or cytologically confirmed R/M NPC who had not received prior systemic therapy and had at least one measurable lesion. Patients were randomized 2:1 to either:
- Tagitanlimab + GP: Tagitanlimab combined with GP chemotherapy for 4 to 6 cycles, followed by tagitanlimab maintenance for up to two years or until disease progression.
- Placebo + GP: Placebo combined with GP chemotherapy for 4 to 6 cycles, followed by placebo maintenance for up to two years or until disease progression. Patients in the placebo group could cross over to receive tagitanlimab upon disease progression.
The primary endpoint was IRC-assessed PFS (time from randomization to disease progression or death from any cause). Secondary endpoints included investigator-assessed progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), overall survival (OS), and safety.
Results
Patient Cohort: 295 patients were randomized (197 to tagitanlimab + GP, 98 to placebo + GP). Baseline characteristics were well-balanced between the groups, including ECOG performance status, baseline EBV DNA expression, and types/locations of recurrence/metastasis.
- PFS:
- Significant improvement with Tagitanlimab + GP (HR = 0.47, 53% lower risk of disease progression/death).
- Median PFS: Not reached (Tagitanlimab + GP) vs. 7.9 mo (PBO + GP).
- Benefit observed across most subgroups.
- ORR: 81% (Tagitanlimab + GP) vs. 74% (Placebo + GP) - Significantly higher.
- DOR: Median 11.7 mo (Tagitanlimab + GP) vs. 5.8 mo (Placebo + GP) - Longer duration.
- OS: Preliminary data shows a favorable trend (HR = 0.62); 12-mo OS: 91% vs. 83%.
- EBV DNA Response: 83% of Tagitanlimab + GP patients converted to undetectable EBV DNA after cycle 1, predicting longer PFS.
Safety Profile
- The incidence of treatment-related adverse events leading to treatment discontinuation was slightly higher in the tagitanlimab + GP group, but the overall profile was comparable.
- Immune-related adverse events (irAEs) were more frequent in the tagitanlimab group (31% vs. 25% in PBO), but most were Grade 1 or 2.
- Common TRAEs (occurring in >30% of patients) included hematological events (e.g., decreased white blood cell count, anemia, neutropenia, decreased platelets), nausea, vomiting, increased ALT/AST, hyponatremia, and hypokalemia. The overall incidence and profile of TRAEs were comparable between the two groups.
- The incidence of serious adverse events was 32% in the tagitanlimab group and 32.7% in the PBO group, demonstrating comparability.
Conclusion
The study concluded that adding tagitanlimab to gemcitabine and cisplatin significantly improves PFSin patients withR/M NPC, with a manageable safety profile and no new safety signals. The mPFS was not reached in the tagitanlimab arm compared to 7.9 mo with GP alone (HR:0.47). The mDoR was also significantly longer (11.7 mo vs. 5.8 mo). Preliminary OSdata shows a favorable trend.
These results have led to the approval of tagitanlimab combined with GP as a first-line treatment for R/M NPC in China, validating PD-L1 inhibitors as a viable first-line option for this indication.
SHR-A1811 in HER2-expressing salivary gland cancers (SGCs): Preliminary Efficacy and Safety Results
Speaker: Dr. Dongmei Ji
Introduction
SGCs is a rare malignancy with diverse histological subtypes. HER2 positivity varies significantly by histology (e.g., 43% in SDC, 39% in CEP), and about 50% of SGCs show HER2 low expression. Systemic therapy for SGC currently remains suboptimal with limited standardized approaches.
Recently HER2-targeted therapies, such as trastuzumab deruxtecan (T-DXd), have shown promise, with T-DXd receiving accelerated FDA approval for HER2-positive solid tumors and demonstrating a 68.4% objective response rate (ORR) in a prior trial. SHR-A1811 is a next-generation ADC that combines trastuzumab with a topoisomerase I inhibitor payload, designed to address these limitations.
Study Design
This was an open-label, single-center, phase 2 Investigator-Initiated Trial(IIT) umbrella study with a Simon's two-stage design.
Key Eligibility Criteria
- Recurrent or metastatic SGC.
- Prior anti-HER2 treatment was allowed.
- ECOG performance status 0-1.
- At least one measurable lesion per RECIST 1.1.
Patient Allocation
- Patients underwent AR and HER2 IHC detection. For IHC 2+ cases, ISH detection was added.
- Arm 1 (HER2 Overexpression/Amplification): Patients with HER2 IHC 3+ or IHC 2+/ISH positive received SHR-A1811 at 4.8 mg/kg IVevery three weeks.
- Arm 4 (HER2 Low Expression): Patients with HER2 IHC 1+ or IHC 2+/ISH negative received SHR-A1811 at 5.6 mg/kg IV every three weeks. (Other arms for HER2-positive and AR-negative/TROP2-negative patients were part of the umbrella trial but not reported in this preliminary analysis).
Endpoints
- Primary Endpoint: ORR.
- Secondary Endpoints: Disease Control Rate (DCR), Progression-Free Survival (PFS), Overall Survival (OS), and safety profiles.
The data cut-off date for this preliminary report was January 21, 2025. Only results from Arm 1 and Arm 4 were presented.
Patient Characteristics
- Arm 1 (HER2 Overexpression/Amplification):
- Median age: 58 years; 70% male; 100% ECOG PS 1.
- HER2 IHC 3+: 83%; IHC 2+/ISH positive: 17%.
- Common histologies: SDC (52%), CEP (13%), others (55%).
- 70% received prior treatment (curative surgery, radiation, systemic therapy). 17% had prior trastuzumab; 9% had prior anti-HER2 ADC.
- Arm 4 (HER2 Low Expression):
- Median age: 56 years; 80% male; 100% ECOG PS 1.
- HER2 IHC 1+: 80%; IHC 2+/ISH negative: 20%.
- Common histologies: SDC (10%), CEP (20%), ACC (20%), others (50%).
- 90% received prior treatment (curative surgery, radiation, systemic therapy). No patients in this arm received prior anti-HER2 therapy.
Common metastatic sites in both arms included lymph nodes, lungs, and bone. The majority of patients had 1-3 metastatic sites.
Results
Efficacy
- Arm 1 (HER2 Overexpression/Amplification):
- ORR: 85.7% (Confirmed ORR: 81%).
- DCR: 100%.
- One patient achieved a Complete Response (CR).
- One patient who had progressed after prior trastuzumab and another anti-HER2 ADC still benefited from SHR-A1811 treatment.
- Neither median OS nor PFS was reached, indicating promising durability. One case study highlighted a patient achieving CR with long-lasting remission.
- Arm 4 (HER2 Low Expression):
- ORR: 30% (Confirmed ORR: 20%).
- DCR: 100%.
- No CRs were observed.
- Neither median OS nor PFS was reached.
Safety
- Overall TRAEs: 97% of patients experienced Treatment-Related Adverse Events (TRAEs).
- Grade 3/4 TRAEs: 48% of patients had Grade 3 or 4 TRAEs.
- Serious TRAEs: Two patients had serious TRAEs.
- Dose Modifications: 42% had dose interruptions; 15% had dose reductions.
- No treatment discontinuation or death due to TRAEs.
- Common TRAEs were primarily hematological toxicities: Neutropenia, leukopenia, anemia, and platelet count decreased.
- Other TRAEs: One patient had infectious pneumonia; one had febrile neutropenia; one patient had Grade 1 interstitial lung disease (ILD)— a relatively low incidence of severe ILD.
Conclusion
The study concluded that SHR-A1811 demonstrated remarkable efficacy and a favorable safety profile in HER2-expressing SGCs, positioning it as a potential practice-changing option.
Darolutamide plus Goserelin for Androgen Receptor-positive Salivary Gland Cancers (SGCs): Results of Phase 2 Study (DISCOVARY)
Speaker: Dr.Susumu Okano
Introduction
Currently, there's no standard treatment for recurrent, metastatic, or unresectable locally advanced SGC. Androgen receptor (AR) overexpression has been identified as a potential molecular target for SGCC. Previous research suggested that combined androgen blockade (CAB) might offer promising clinical activity in AR+ SGC, but no AR-targeted drug is currently approved for this indication.
Darolutamide is a next-generation AR signaling inhibitor known for its strong binding affinity and minimal partial agonist effect, leading to robust AR inhibition. Its unique pharmacokinetics, including low blood-brain barrier penetration, help minimize central nervous system (CNS) side effects. It has shown efficacy in prostate cancer and its favorable tolerability makes it suitable for combination therapy with LHRH agonists like goserelin.
The DISCOVARY study previously investigated darolutamide monotherapy, which showed some limited activity (Objective response rate (ORR) of 20.8%). This presentation focuses on the combination phase of the study.
Study Design
This multicenter phase 2 study aimed to evaluate the efficacy and safety of darolutamide in combination with goserelin.
Key Eligibility Criteria
- AR-positive SGC (confirmed by central assessment).
- Measurable lesions.
- Good performance status (ECOG PS 0-1).
- Adequate organ function.
Treatment Regimen
- Darolutamide: 1200 mg/day orally.
- Goserelin acetate: 3.75 mg every four weeks subcutaneously.
Treatment continued until disease progression or unacceptable toxicity.
Endpoints
- Primary Endpoint: Objective Response Rate (ORR) by Independent Central Review (ICR).
- Secondary Endpoints: ORR by investigator, Disease Control Rate (DCR), Clinical Benefit Rate (CBR), Progression-Free Survival (PFS), Overall Survival (OS), and Quality of Life (QoL).
Demographics & Disease Characteristics (n=33)
- Median age: 63 years.
- Majority were male with good performance status (ECOG PS 0-1).
- Histology: Predominantly salivary ductal carcinoma (32/33 cases).
- AR positivity: About 90% of cases had AR positivity of 70% or higher.
- Metastasis: Many patients had metastasis, mainly to the lungs and lymph nodes.
- Primary sites: Mainly parotid or submandibular gland.
- Prior treatment: About half had received previous chemotherapy.
- AR concordance rate between local and central assessment was 94%. Discordances were due to differences in tissue evaluated or interpretation.
Results
Efficacy
- ORR by ICR (Primary Endpoint):
- 2%
- This result met the predefined hypothesis.
- More than half of the patients experienced tumor shrinkage.
- Duration of Response: Spider plots showed early tumor shrinkage in responders and prolonged tumor control in many cases, emphasizing stable clinical benefit.
- PFS: Median PFS was 13.1 mo.
- OS:
- Median OS was not reached at the data cutoff.
- Median follow-up was 13.7 mo.
These findings suggest that the darolutamide combination therapy led to early and durable tumor control and promising overall survival.
Response Based on AR Positivity
Out of 31 patients, 29 had AR positivity of 70% or higher. The 2 patients with less than 70% AR positivity did not respond to the treatment. While the sample size is small, this suggests a potential cutoff for efficacy.
Response Based on HER2 Status
The study did not investigate HER2 status. However, among the 6 patients who received prior HER2 treatment, about half responded to the darolutamide-goserelin combination, suggesting potential efficacy even in previously HER2-treated patients.
Safety
- Overall Adverse Events (AEs): Most AEs were Grade 1 or 2.
- Grade 3 or Higher AEs: Occurred in only 6 patients (18.2%). Included one case each of neutropenia, hypercalcemia, infection, hypersensitivity, atrial fibrillation, drug rash, and jawbone necrosis.
- Treatment-Related Fatal AEs: None reported.
- Serious AEs leading to suspicion of drug-relatedness: 9.1% (hypersensitivity, fever, disseminated intravascular coagulation).
- Overall: The combination therapy was generally well tolerated.
Conclusion
The DISCOVARY study, a prospective multicenter trial of combined androgen blockade (CAB) in SGC, successfully met its primary endpoint.
- Darolutamide plus goserelin demonstrated clinically meaningful efficacy and a favorable safety profile.
- Given the potential of chemotherapy to reduce patients' quality of life, this combination therapy may be a compelling option before initiating chemotherapy for AR+ SGC patients.
ASCO 2025, May 30 – June 3, Chicago