TIOTROPIUM 2018: Unfolding 15 Years of Evolution
Focus of Tiotropium
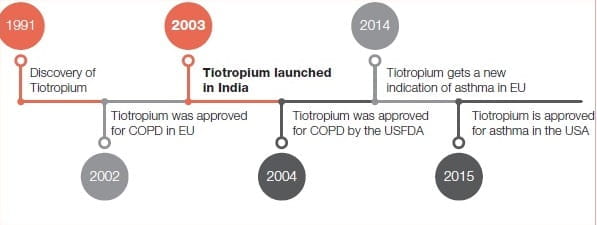
Journey of Tiotropium
From Datura to Tiotropium2
Anticholinergics originate from botanical preparations of the deadly nightshade family (Solanaceae), which have been used for hundreds of years in many cultures worldwide. Datura is a genus of the Solanaceae family and it grows worldwide. When burnt the roots, stems and seeds of this plant release an aerosol of potent alkaloids, one of which is the antimuscarinic compound, atropine.
It was the inhalation of this medicinal smoke that was a treatment for obstructive airways disease for many centuries. Derived from atropine-containing plants, anticholinergics work by inhibiting the parasympathetic-cholinergic system and have been in use since the Egyptian and Greek civilizations.
Atropine-based agents became the standard bronchodilators for respiratory disorders; however, the unpleasant side effects of the emerging adrenergic drugs in the 1920s and theophyllines in the next decade led to a decline in the use of these agents for many years.
Following studies describing the role of the parasympathetic system in controlling the calibre of the airways, there was an emerging interest in anticholinergic therapies. This led to the recognition that inhibiting the parasympathetic-cholinergic system could be an alternative therapeutic approach to improving airflow and revived an interest in using anticholinergics. Available anticholinergics include ipratropium bromide, oxitropium, and most recently tiotropium, all of which work by blocking the muscarinic receptors for the neurotransmitter, acetylcholine, released from cholinergic nerve endings in the airways.
Whilst oxitropium and, specifically, ipratropium have shown benefits, tiotropium has an edge over its precursors in terms of its selectivity and clinical effects, specifically in terms of the symptoms exhibited by patients.
Pharmacology of Tiotropium 3,4
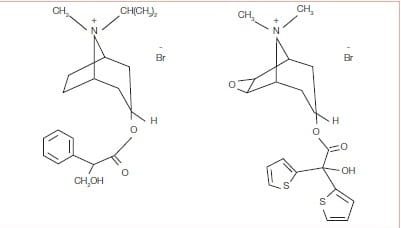
Tiotropium has a quaternary ammonium structure and is derived from Ipratropium bromide (Figure 1).
Tiotropium is a potent muscarinic receptor antagonist, with a prolonged duration of blockade. Tiotropiu m bromide dissociates more slowly from M and M receptors than M receptors and, forth is reason, has kinetic selectivity
Studies using recombinant receptors have shown that tiotropium has a much slower rate of dissociation from M muscarinic receptors than Whilst oxitropium and ipratropium and conclude that this feature determines the difference in duration of action between the drug
Whilist oxitropium and specifically, ipratropium have shown benefits, tiotropium has an edge over its precursors in terms of its selectivity and clinical effects, specifically in terms of the symptoms exhibited by patients
Indeed, it has been demonstrated that the slow dissociation of tiotropium bromide from target protein is a key factor in its long duration effect. Though the two compounds have similar pharmacokinetic profiles, tiotropium’s duration of action is more than 24 hours, while ipratropium action lasts only 6 hours. Because of its sustained activity, once-daily administration is possible.
Tiotropium bromide binds to muscarinic receptors with high affinity and is approximately 10 times more potent than ipratropium bromide in binding to human lung muscarinic receptors. Ipratropium bromide and oxitropium bromide are non-selective blockers; consequently, they block M receptors, thereby increasing acetylcholine (ACh) release at the nerve endings, which may reduce the degree of blockade or the duration of action.
Pharmacokinetics of Tiotropium5
Tiotropium demonstrates linear pharmacokinetics in its therapeutic range after dry powder inhalation.
Onset and duration
The time to a clinically significant increase in FEV1 after inhalation is 30 minutes. The time to the peak increase in FEV1 after inhalation is 1.5 to 3 hours. Tiotropium’s duration of action is at least 24 hour.
Absorption
The absolute bioavailability following dry powder inhalation is 19.5%. Tiotropium is poorly absorbed from the gastrointestinal tract, with an absorption of approximately 10–15%. Maximum plasma concentrations were observed 5 minutes after inhalation.
Distribution
The volume of distribution for tiotropium is 32 L/kg, and it is 72% bound to plasma proteins. The peak plasma level at steady state, measured 5 minutes after inhalation of an 18-mcg dose, was 17 to 19 mcg/mL.it decreased rapidly in a multicompartment manner. Steady-state trough plasma concentrations were 3 to 4 mcg/mL. The concentration of tiotropium in the lungs is unknown at the present time. After long-term once-daily inhalation by COPD patients, pharmacokinetic steady state was reached after 2 to 3 weeks with no accumulation thereafter.
After long term once- daily inhalation by COPD patients, the pharmacokinetic steady state was reached after 2 to 3 weeks with no accumulation thereafter
Metabolism
The extent of tiotropium biotransformation is small. The ester tiotropium is non-enzymatically cleaved to the inactive alcohol N-methylscopine and the inactive acid compound, dithienylglycolic acid.
Elimination
The terminal elimination half-life is 5 to 6 days. After dry powder inhalation, 14% of the dose is eliminated via urinary excretion. The remainder is mainly non-absorbed drug in the gastrointestinal tract, which is eliminated via the faeces. The renal clearance of tiotropium exceeds the creatinine clearance, indicating secretion into the urine.
Tiotropium in COPD
COPD and the Place of Anticholinergics
A combination of genetic, constitutional, familial, behavioural, sociodemographic and environmental factors predispose patients to COPD with cigarette smoking being the most common cause.3
These factors damage the airways and impair the defense and repair mechanisms, leading to structural narrowing of the airways, which is predominantly irreversible due to a combination of fibrosis, mucus hyperplasia and alterations in the vagal bronchomotor tone.
Loss of elastic recoil in the lungs caused by parenchymal destruction characterizes the emphysemic component.
Increased resistance in the airways as a result of airway inflammation increases the thickness of the airway walls, and narrows the airway, which reduces the driving pressure; the obstructive element.
There is also excessive mucus secretion increased by goblet cell proliferation; which characterizes the bronchitic component.3
A combination of genetic, constitutional, familial, behavioural, sociodemographic and environmental factors predispose patients to COPD
For patients this means declining lung function, breathlessness and other symptoms, exacerbations and reductions in the health status or (HRQoL).
COPD – A Multicomponent Disease: The Clinical Focus
The airflow obstruction of COPD, as expressed by FEV1, is by definition only partially reversible. In a paradoxical way, this defining physiology has been used as the outcome to determine the effectiveness of interventions. It is no surprise that the lack of a large response in FEV1 to different therapies has resulted in an undeserved nihilism
There is increasing evidence that independent of the degree of airflow obstruction, lung volumes are important in the genesis of the symptoms and limitations of patients with more advanced disease. A series of studies have demonstrated that dyspnoea perceived during exercise, including walking, more closely relates to the development of dynamic hyperinflation than to changes in the FEV1. Further, the improvement in exercise brought about by several therapies, including bronchodilators, oxygen, lung volume reduction surgery (LVRS) and even rehabilitation is more closely related to delaying dynamic hyperinflation than improving the degree of airflow obstruction.3
Indeed, dyspnoea measured with simple tools such as the modified Medical Research Council scale, the body mass index and the timed 6-minute walk distance (6MWD) are all better predictors of mortality than the FEV1
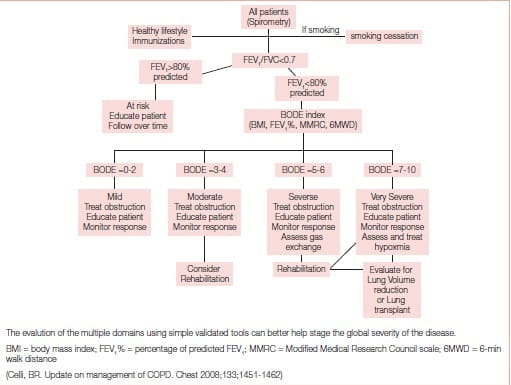
The BODE (body mass index, airflow obstruction, dyspnoea, exercise capacity) index, also responsive to exacerbations, and more importantly acting as a surrogate marker of future outcomes after interventions, may help clinicians determine the comprehensive severity of disease better.5
It has also been shown that hyperinflation, expressed as the inspiratory capacity/ total lung capacity ratio, predicted survival better than the FEV1. The importance of lung volume as determinant of outcomes provides not only with new insights into the pathogenesis, but also opens the door for new imaginative ways to alter lung volumes and perhaps, impact disease progression.5
It is now accepted that COPD may be associated with important systemic expressions in patients with more advanced disease. Perhaps as a consequence of a persistent systemic inflammatory state or due to other yet unproven mechanisms such as imbalanced oxidative stress or abnormal immunologic response, many patients with COPD may have decreased fat-free mass, impaired systemic muscle function, anaemia, osteoporosis, depression, pulmonary hypertension and cor pulmonale, all of which are important determinants of outcome.5
The BODE index, acting as a surrogate marker of future outcomes after interventions, may help clinicians determine the comprehensive severity of disease better
The incorporation of these variables into the multidimensional BODE index predicts survival even better.5
Based on the multidimensional nature of the disease and the availability of multiple effective therapies, the proposed approach shown in Figure 2 may help clinicians evaluate patients comprehensively and choose therapies other than the current approach of using primarily the percentage of predicted FEV1 from reference values.5
Few treatments have been developed specifically for patients with COPD. Treatments were evolved from asthma treatments through a tendency to treat the two diseases as one, because of a misunderstanding of the pathogenesis of the diseases and an erroneous view that COPD treatment can be considered as palliative rather than curative or restorative. This was reinforced by insufficient evidence to conclude that any specific.
Inhaled therapy is the cornerstone of treatment comprising anticholinergics and beta2 -agonists pharmacotherapeutic agents significantly altered the outcome of the disease process.5
A growing evidence base now supports the use of bronchodilators in COPD to improve function, exercise tolerance and health status. Inhaled therapy is the cornerstone of treatment comprising anticholinergics which relax airway smooth muscle by blocking cholinergic tone (the primary reversible component in COPD), and beta2-agonists which are non-specific functional bronchodilators that work via the sympathetic pathway. There is increased evidence of a greater response to anticholinergics than beta 2 -agonists.5
Tiotropium in the Clinic
Dynamic Hyperinflation and Exercise Performance
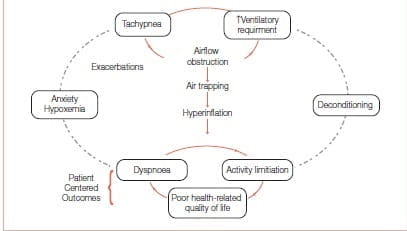
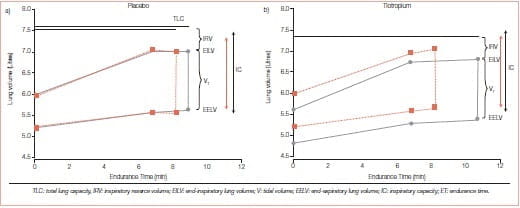
FEV1 has served as an important diagnostic measurement of COPD but has not been found to correlate with patient-centred outcomes such as exercise tolerance, dyspnoea or HRQoL. It has not helped us understand why some patients with severe FEV1 impairment have better exercise tolerance compared with others with similar FEV1 values. Hyperinflation, or air trapping caused by expiratory flow limitation, cause operational lung volumes to increase and even approach the total lung capacity (TLC) during exercise (Figure 3).
Figure 3: Air trapping links the pathophysiology and patient-centred outcomes in COPD
Some study findings suggest that a dyspnoea limit is reached when the end inspiratory lung volume encroaches within approximately 500 mL of TLC. The resulting limitation in daily physical activity establishes a cycle of decline that includes physical deconditioning (elevated blood lactic acid levels at lower levels of exercise) and worsening dyspnoea. Hyperinflation is reduced by long-acting bronchodilators that reduce airways resistance. The deflation of the lungs, in turn, results in an increased IC.7
Tiotropium increases the IC, the 6MWD, and the cycle exercise endurance time, and it decreases isotime fatigue or dyspnoea.8
To test the hypothesis that the use of tiotropium would be associated with sustained reduction in lung hyperinflation and, thereby, would improve exertional dyspnoea and exercise performance in patients with COPD, a randomized, double-blind, placebo-controlled, parallel-group study was conducted in 18 patients (FEV 44 ± 13% pred); 96 patients received 18 mcg tiotropium and 91 patients received placebo once daily for 42 days
- Spirometry, plethysmographic lung volumes, cycle exercise endurance and exertional dyspnoea intensity at 75% of each patient’s maximal work capacity were compared.
- On day 42, the use of tiotropium was associated with the following effects at pre-dose and post-dose measurements as compared to placebo:
- Vital capacity and IC increased, with inverse decreases in residual volume and functional residual capacity.
- Tiotropium increased post-dose exercise endurance time by 105 ± 40 seconds (21%) as compared to placebo on day 42
- At a standardised time near end-exercise (isotime), IC, tidal volume and minute ventilation all increased, whilst dyspnoea decreased by 0.9 + 0.3 Borg scale units (Figure 4).
In this study, the use of tiotropium was associated with sustained reductions of lung hyperinflation at rest and during exercise. The resultant increases in the IC permitted greater expansion of the tidal volume and contributed to improvements in both exertional dyspnoea and exercise endurance.
To further understand the duration of improvements in reduction of lung hyperinflation another randomized, double-blind, placebo-controlled, parallel group study was conducted in 261 COPD patients (mean age62.5 ± 7.4 years [± SD]; 189 men and 72 women; mean FEV1:1.2 ± 0.4 L [43 ± 12.7% predicted]).9
On day 0 (first dose), day 21, and day 42 of treatment, pulmonary function tests were performed before and 100 minutes after dosing, followed by a constant work rate cycle ergometry test (75% maximum work capacity) to symptom limitation at 2.25 hours after dosing. On day 42, an additional constant work rate cycle ergometry test was performed at 8 hours after dosing.
Tiotropium reduced lung hyperinflation at rest and during exercise, reduced exertional dyspnoea, and improved symptom-limited exercise tolerance in COPD patients. Furthermore, this study shows that this improvement is present at 2.25 hours and at 8 hours post dosing after 6 weeks of treatment.
In this study. The use of tiotropium was associated with sustained reductions of lung hyperinflation at rest and during exercise.
Improvement of Walking Endurance 10 in COPD
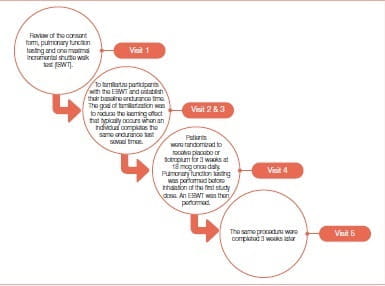
Bedard et al. conducted a single-center, double-blind, randomized study, which required five visits (Figure 5) to the research facility at a tertiary care hospital that is the referral hospital for respiratory diseases in the eastern part of the province of Quebec, Canada. An endurance shuttle walk test (ESWT) is a simple field exercise test that was developed initially to asses exercise capacity in COPD patients. Endurance walking capacity was determined with the ESWT while peak walking capacity was determined by the incremental shuttle walk test (ISWT).
Subjects included in this study were clinically stable patients with COPD, who were aged ≥50 years, had a forced expiratory volume in 1 second (FEV1 ) ≤70% predicted, a FEV /forced vital capacity (FVC) <70% and a smoking history of >10 pack-years.
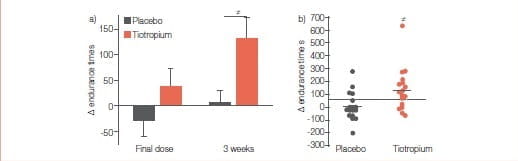
(b) Individual changes in walking endurance time at 3 weeks. The grey and black horizontal lines in (b) represent the group mean change seen in the placebo and tiotropium groups, respectively, while the dashed line represents the minimal important difference for the ESWT (65 seconds).
The between-group difference in the improvement in walking endurance time was statistically significant at 3 weeks, whereas it was not different after a single dose (p=0.12). #: between-group comparison p=0.017.
Results
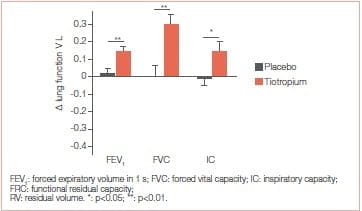
At 3 weeks, tiotropium significantly improved walking endurance time, with a mean ± SD between-group difference of 128±141 seconds (p=0.017). Also, trough values of FEV and FVC were significantly improved with tiotropium. Improvement in walking capacity with tiotropium was larger with the ESWT (32%) than that reported for the ISWT (11%) Tiotropium for 3 weeks resulted in a greater walking endurance in patients with COPD. Improvements in FEV, maximal ventilation and tidal volume may contribute to this enhanced exercise capacity
The 6-minute Walk Test
In clinical practice, the 6-minute walk test (6MWT) is commonly used to assess changes in functional exercise capacity in COPD patients following PR with the primary outcome reported being the distance walked during the test (i.e., 6MWD). The 6MWD has demonstrated validity, reliability after one familiarization test and the capacity to detect changes following PR. In addition to assessing the outcomes of PR, the 6MWD may be used to quantify the magnitude of a patient’s disability, prescribe a walking programme, identify patients likely to benefit from a rollator and to identify the presence of exercise-induced hypoxaemia.
The effect of a single dose of tiotropium on the exercise capacity of stable COPD patients was assessed with the 6MWT.11 The study was a randomized, placebo-controlled, double-blind, cross-over study. All together 44 stable COPD patients with moderate to severe airway obstruction were selected according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria. The regular anticholinergic therapies of the patients were interrupted 1 week before the test. In the morning hours of the first day, half the group was given one capsule (18 mcg) of tiotropium and the other half was given placebo as inhalation, using the Handi Haler device.
The exercise capacity, therefore, improved in moderate-to-severe COPD patients by using a single dose tiotropium inhalation.
Before and 120 minutes after the medication, the 6MWT was performed. Oxygen saturation, modified Borg dyspnoea ratings, blood pressure and heart rate were recorded before and after the test. The same procedure was repeated at the same time on the third day but this time the patients were given placebo if they used tiotropium on the first day and vice versa. Before using tiotropium or placebo, there was no difference between 6MWDs.
The 6MWD after the use of tiotropium (429.3 ± 70.6 meters) was significantly longer than that after the use of placebo (414.7 ± 74.6 meters).
The changes in the Borg dyspnoea ratings and arterial oxygen saturation values with tiotropium and placebo use were not significant. The exercise capacity, therefore, improved in moderate-to-severe COPD patients by using a single dose tiotropium inhalation.
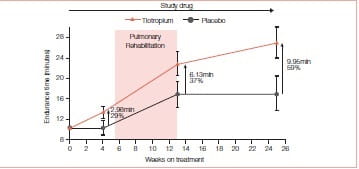
Pulmonary Rehabilitation
Pulmonary rehabilitation (PR) improves the exercise tolerance in COPD patients. Ventilatory mechanics improvements from tiotropium could permit enhanced ability to train the muscles of ambulation and therefore, augment the exercise tolerance benefits of PR.
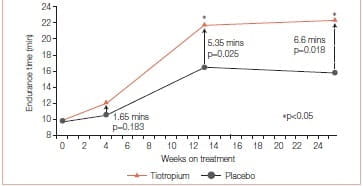
To assess this, two trials were conducted. In one randomized, double-blind, placebo controlled trial (tiotropium, n = 47; placebo, n = 44), tiotropium (18 mcg qd) was administered to COPD patients participating in 8 weeks of PR (treadmill training three times a week;≥30 minutes per session) at 17 sites. The study drug was administered 5 weeks prior to, 8 weeks during, and 12 weeks following PR. The primary end point was treadmill walking (0% incline) endurance time at 80% of the maximum speed attained in an initial incremental test. The transition dyspnoea index (TDI), St. George’s respiratory questionnaire (SGRQ) and rescue salbutamol use were secondary end points.12
Mean age of the 93 participants was 67 years, 57% were men and mean FEV1 was 0.88 L (34% predicted).
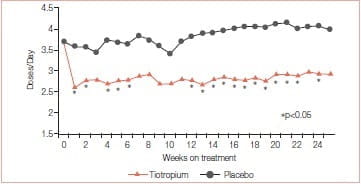
Mean endurance time (ET) differences (tiotropium minus placebo) prior to PR, at the end of PR, and 12 weeks after PR were 1.65 minutes (p = 0.183), 5.35 minutes (p = 0.025), and 6.60 minutes (p = 0.018), respectively. Mean TDI focal scores at the end of PR were 1.75 for tiotropium and 0.91 for placebo (p >0.05). At 12 weeks after PR, TDI focal scores were 1.75 for tiotropium and 0.08 for placebo (p < 0.05). Relative to placebo, tiotropium improved the SGRQ total scores by 3.86 at the end of PR and 4.44 at 12 weeks after PR (p > 0.05). Mean salbutamol use declined following PR plus tiotropium, compared to PR alone (p≤ 0.05 for 17 of 25 weeks) (Figures 8 & 9).
This study concluded that tiotropium in combination with PR improved endurance of a constant work rate treadmill task and produced clinically meaningful improvements in dyspnoea and health status compared to PR alone. Improvements with tiotropium were sustained for 3 months following completion of PR.
Tiotropium in combination with PR produced clinically meaningful improvements in dyspnoea and health status compared to PR alone.
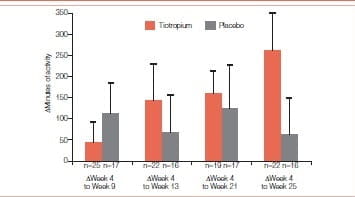
In another similar study, 13 COPD patients participating in 8 weeks of PR were studied in a randomized, double-blind, placebo-controlled trial of tiotropium 18 mcg daily (tiotropium = 47; placebo = 44). The study drug was administered for 5 weeks prior to, 8 weeks during and 12 weeks following PR. Patients completed a questionnaire documenting participation in pre-defined activities outside of PR during the 2 weeks prior to each visit. Patients who submitted an activity questionnaire at week 4 and on at least one subsequent visit were included in the analysis. For each patient, the number of sessions was multiplied with the duration of each activity and then summed up to give the overall activity duration.
Patients receiving tiotropium reported, on average, 262 minutes more exercise at the end of the study whereas patients receiving placebo reported a gain of only 60 minutes.
Patients (n = 46) had a mean age of 67 years, with a mean baseline FEV1 of 0.84 L (33% predicted). Mean (SE) increase in duration of activities (minutes during the 2 weeks prior to each visit) from week 4 (prior to PR) to week 13 (end of PR) was 145 (84) minutes with tiotropium and 66 (96) minutes with placebo. The increase from week 4 to week 25 (end of follow-up) was 262 (96) and 60 (93) minutes for the respective groups. Increases in activity duration from week 4 to weeks 17, 21, and 25 were statistically significant with tiotropium (Figures 10 & 11)
Figure 10: Mean (SE) difference in duration of activities from week 4 (prior to PR) to subsequent visits as reported through the activity questionnaire
Patients receiving tiotropium reported, on average, 262 minutes more exercise at the end of the study whereas patients receiving placebo reported a gain of only 60 minutes.
Patients receiving tiotropium continued to increase their measured exercise endurance during the 12-week period following PR.
The authors concluded that tiotropium appears to amplify the effectiveness of PR as seen by increases in patient self-reported participation in physical activities.
Breathlessness
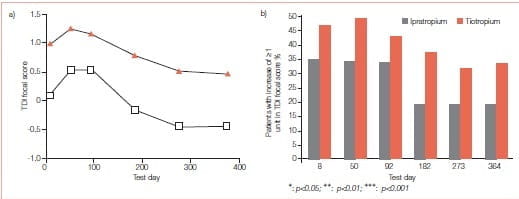
The effect of tiotropium on dyspnoea has been measured by Mahler’s TDI for the dyspnoea incurred by activities of daily living in COPD. The improvement in TDI in patients receiving tiotropium has been shown to be statistically higher than placebo and ipratropium at all time points over a 12 month period and tends to increase as treatment progresses. In the studies comparing salmeterol versus tiotropium, the improvement in TDI with tiotropium was greater than with salmeterol.
The exercise capacity, therefore, improved in moderate-to-severe COPD patients by using a single dose tiotropium inhalation.
Patients with stable COPD (age 65.2 ± 8.7 yrs (mean ± SD), n=921) were enrolled in two identical randomized double-blind placebo-controlled 1 year studies. Patients inhaled tiotropium 18 mcg or placebo once daily as a dry powder. Changes in dyspnoea were measured using the TDI.
Significant improvements in breathlessness compared to baseline, as assessed using the TDI focal score, were achieved by the first assessment (day 50) and maintained for the entire year in the tiotropium group compared to placebo. Differences ranged from 0.8 ± 0.2 to 1.1 ± 0.2 (p<0.001 at all-time points) (Figure 9). The proportion of patients who achieved a score of ≥1.0 (corresponding to a clinically important difference) in TDI focal score was significantly greater in the tiotropium group at all five assessment points (42-47%) than in the placebo group (29-34%) (p<0.0l) (Figure 12).
COPD symptom scores showed statistically significant improvements for shortness of breath and wheezing (p<0.05), compared with placebo.
Tiotropium is an effective, once-daily bronchodilator that reduces dyspnoea and COPD exacerbation frequency and improves health status. This suggests that tiotropium will make an important contribution to COPD therapy.14
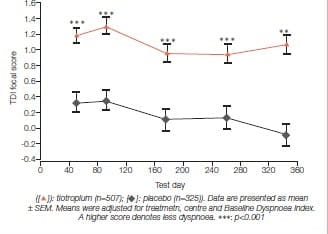
Improvements in dyspnoea was assessed in two identical 1-year randomised double-blind double-dummy studies of tiotropium 18 mcg od (n=356) compared with ipratropium 40 mcg q.i.d. (n=179).
Tiotropium significantly improved all three components of the TDI, as well as the focal score, on all test days compared to ipratropium.
The baseline dysnoea index (BDI) focal scores (mean± SEM) for the tiotropium group (7.13 ± 0.14) and ipratropium group (7.41 ± 0.19) were comparable (p<0.05).
Tiotropium significantly improved all three components of the TDI, as well as the focal score, on all test days compared to ipratropium. The differences in TDI focal score between the tiotropium and ipratropium groups at 9 and 12 months were 0.97 ± 0.25 and 0.90 ± 0.26, respectively (Figure 13a). The proportion of patients who achieved a clinically meaningful difference in TDI focal score (improvement of ≥ 1unit) at 1 year was significantly greater in the tiotropium group (31%) than in the ipratropium group (18%, p=0.004) (Figure 13 b).14
A 6-month, randomized, placebo-controlled, double-blind, double-dummy, parallel-group study of tiotropium, 18 mcg once daily via dry-powder inhaler, compared with salmeterol, 50 mcg bid via metered- dose inhaler, was conducted in patients with COPD. TDI was one of the efficacy measures assessed.16
BDI focal scores were comparable between groups (tiotropium, 6.65; salmeterol, 6.62; and placebo, 6.21), and were consistent with a moderate degree of dyspnoea.
At 6 months, the improvement in TDI focal scores above placebo was 1.02 U for tiotropium (p=0.01) and 0.24 U for salmeterol (p=0.56). Tiotropium was superior to salmeterol in improving TDI focal score (difference, 0.78 U; p < 0.05). The proportion of patients within each group achieving a 1 U change was 42%, 35%, and 26% for the tiotropium, salmeterol, and placebo groups, respectively. The differences were significant for tiotropium vs. placebo (p·< 0.01) but not for salmeterol vs. placebo or for tiotropium vs. salmeterol. Tiotropium appeared to improve dyspnoea consistently over time, where there appeared to be a deterioration from the middle to the end of the trial for the other groups.
Hyperinflation and Treadmill Exercise Tolerance in Mild-to-Moderate COPD Patients17
Increasing exercise tolerance and enhancing lung function in mild-to-moderate COPD is critical in the therapy of COPD patients.
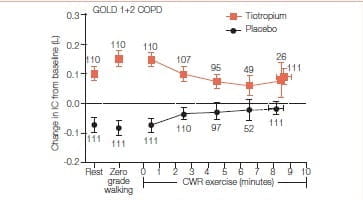
The study was done to determine effects of tiotropium on lung hyperinflation and exercise tolerance in patients with GOLD 1 and GOLD 2 COPD who experienced inspiratory capacity decrease greater than or equal to 100 mL. during incremental and constant work rate treadmill exercise.
It was a 22-week, randomized, double-blind, two-period crossover study that assessed the efficacy of once-daily tiotropium bromide (18 mg) versus placebo in patients with GOLD 1 (n=48) COPD and GOLD 2 (n=78) COPD.
Primary endpoint was between-group difference in inspiratory capacity at isotime (i.e., at the time the shortest test ended) during constant work rate treadmill exercise from baseline to the end of a 6-week treatment period. Key secondary endpoints included differences in exercise duration and exertional dyspnea.
Results
- Tiotropium led to significant improvement in resting inspiratory capacity versus placebo in the overall (P<0.0001), GOLD 1 (P=0.0183), and GOLD 2 (P<0.0001) groups.
- Isotime inspiratory capacity was significantly enhanced during exercise in the overall (P=0.0087) and GOLD 2 (P=0.0494) groups after tiotropium versus placebo.
- Tiotropium significantly enhanced exercise duration in the GOLD 2 group (P=0.0070) but not in the GOLD 1 or overall patient groups. In the overall group, increase in exercise duration seen with tiotropium was well correlated with the increase in isotime inspiratory capacity (r=0.463, P<0.0001).
|
Variables |
GOLD Group |
Patients, n |
Tiotropium* |
Placebo* |
Tiotropium vs. Placebo† |
P Value |
|
Change in resting |
1 + 2 |
111 |
0.079 ± 0.021 |
-0.048 ± 0.021 |
0.127 ± 0.024 |
<0.0001 |
|
IC before |
1 |
43 |
0.021 ± 0.034 |
-0.071 ± 0.034 |
0.092 ± 0.038 |
0.0183 |
|
Exercise, L |
2 |
68 |
0.116 ± 0.027 |
-0.034 ±0.027 |
0.150 ± 0.031 |
<0.0001 |
|
Isotime‡ change |
1 + 2 |
111 |
0.068 ± 0.020 |
0.004 ±.0020 |
0.065 ± 0.024 |
0.0087 |
|
in IC during |
1 |
43 |
0.059 ± 0.034 |
-0.007 ± 0.034 |
0.066 ± 0.040 |
0.0977 |
|
CWR exercise |
2 |
68 |
0.074 ± 0.027 |
0.010 ± 0.027 |
0.064 ± 0.032 |
0.0494 |
|
Test, L |
|
|
|
|
|
|
Definition of abbreviation: CI = confidence interval; CWR = constant work rate; GOLD = Global initiative for Chronic Obstructive Lung Disease; IC = inspiratory capacity.
*Adjusted mean ± SE change from baseline.
†Adjusted treatment difference mean ± SE change from baseline.
‡Isotime was defined as the shortest end-exercise time that was reached during both the post-treatment and corresponding baseline constant work rate exercise tests.
Resting and exercise hyperinflation were enhanced by bronchodilator therapy with tiotropium in the overall GOLD 1 plus GOLD 2 COPD groups. Exercise tolerance was enhanced in GOLD 2, but not GOLD 1 COPD.
Effect on Night-time Awakening18
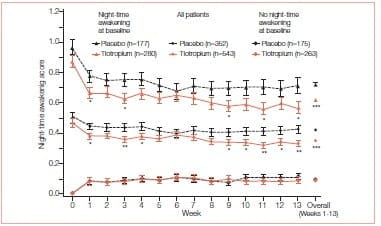
Quality of sleep is often poor in patients with COPD, but these night- time symptoms are a forgotten dimension of COPD and, many times, go unreported by patients. Some studies report that night-time awakenings because of COPD symptoms decrease health status. Calverly et al. conducted a twin, multicenter, double-blind, randomized, placebo- controlled, parallel-group trial in which patients with stable moderate-to-severe COPD (pre-bronchodilator FEV1 ≤65% of predicted normal) were randomized to tiotropium (18 mcg, n=550) or placebo (n=371) and were followed for 13 weeks.
Night-time symptoms are a forgotten dimension of COPD and, many times, go unreported by patient
During a 2-week, pre-treatment baseline period and for 13 weeks on treatment, daily recording of self- reported night-time awakenings due to COPD symptoms, rescue medication (albuterol) use, and morning and evening peak expiratory flow rate (PEFR) was done. Health-related quality-of-life (HRQoL) and energy– fatigue questionnaires were completed at baseline and during treatment. For each night, patients scored as follows: 0 for no awakenings; 1 for one awakening; 2 for two or three awakenings; and 3 for being awake for most of the night. These scores were used to calculate a weekly mean for each patient.
Over the 13-week treatment period, tiotropium was associated with fewer night-time awakenings, with mean±standard error overall awakening scores per week of 0.356±0.006 compared with 0.421±0.007 for placebo (p<0.001); means were significantly lower for tiotropium versus placebo in patients with baseline awakenings (p<0.001), but not for those without baseline awakenings.
COPD-related night-time awakenings were associated with increased nocturnal rescue medication use and lower HRQoL in both treatment groups. After the start of treatment, tiotropium decreased use of rescue medication compared with placebo, and morning and evening PEFR were higher for the tiotropium group with respect to placebo.
Tiotropium and Rescue Medication17
The paper concluded that tiotropium is associated with decreased COPD-related night-time awakenings. Night-time awakenings are associated with increased nocturnal rescue medication use and might serve as a marker of symptom control in COPD patients.
Effect of Tiotropium on Exacerbations
COPD exacerbations are episodes in the natural course of the disease characterized by worsening of respiratory symptoms. Exacerbation frequency is related with disease progression, quality of life and management of COPD. Events of exacerbations are indicative of the health status in COPD patients. In most cases, exacerbations are triggered by infections, especially rhinovirus, the cause of the common cold. Hence, prevention of exacerbations in COPD becomes very crucial for disease management.
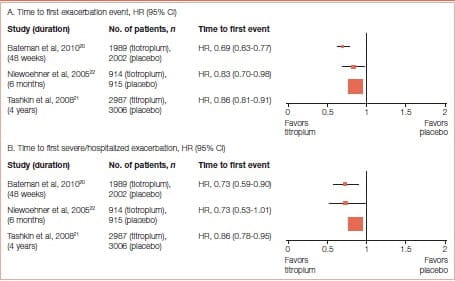
Long-acting bronchodilators effectively manage symptom relief and are the recommended choice of treatment in COPD. Tiotropium is beneficial in reducing exacerbation risk in COPD. Many trials of tiotropium have demonstrated its efficacy in reducing exacerbations. Halpin et al 19 published a systematic review on the effect of tiotropium on COPD exacerbations in 2016. The outcome of this comprehensive review (Figure 17) manifested the benefits of tiotropium in reducing the risk of exacerbations.
Tiotropium is beneficial in reducing exacerbation risk in COPD
Tiotropium and Frequent Exacerbator Phenotype
Recently, patients with frequent exacerbations have been recognized as a distinct clinical subgroup, which is associated with poorer health outcomes. Tiotropium maneuvered a protective role for moderate exacerbations in patients at risk of frequent exacerbations. Given the impact of exacerbations on patient health and on the management of disease, it is crucial to target COPD patients for optimal treatment.
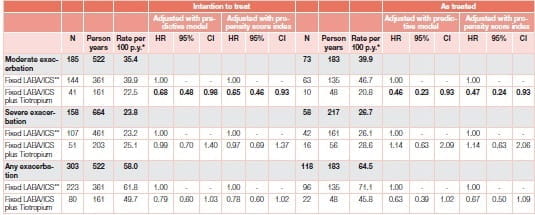
A study23 by Ferroni et al. included a large cohort of individuals (N=5,717) discharged with a diagnosis of COPD exacerbation to represent patients with moderate–severe COPD, and evaluated the effect of tiotropium on COPD exacerbations over the following 12 months. Analysis suggests a protective role for moderate exacerbation in the sub-group of COPD patients with a history of exacerbations over 12 months. This new-user cohort study was based on administrative data from three Italian regions where adults hospitalized for COPD from 2006 to 2009 were identified and newly prescribed a fixed ICS/LABA combination (double therapy). The patients were further classified according to whether tiotropium was also prescribed (triple therapy), using intention-to-treat and as-treated approaches, and were followed for 1 year. COPD exacerbations were measured as outcomes (Figure 18).
In the sub-cohort of patients (n=945) with previous exacerbations, tiotropium add-on to ICS/LABA was significantly associated with reduced risk of moderate exacerbations, compared to double therapy (Table 2).
The Uplift Trial24,25,26,27,28
In COPD, the rate of decline in FEV1 and progression to disability and death are accelerated. COPD management goals include preventing or slowing the progressive loss of lung function, relieving symptoms, improving exercise tolerance and the patient’s health status, preventing and treating exacerbations and complications, minimizing the side effects of the treatment, and reducing mortality.
Although lung function is important for the diagnosis of COPD and classification of its severity, clinicians and patients are also very interested in the symptoms, ability to function and general well-being (health status). Consequently, increasing attention is being given to these patient-centred outcomes.
UPLIFT was designed to evaluate the long-term effects of tiotropium on the rate of decline in lung function and health status as a well as the frequency of exacerbations
Understanding the Potential Long-Term Impacts on Function with Tiotropium (UPLIFT)-is a 4-year landmark trial that examined the effects of maintenance treatment with Tiotropium on the yearly rate of decline in trough FEV1 and the yearly rate of decline in FEV190 minutes after maximal or near-maximal bronchodilator administration and health status as well as the frequency of exacerbations in patients with COPD
The primary endpoints
The two co-primary end points were the yearly rate of decline in the mean FEV1
- Before the use of a study drug and short-acting bronchodilators in the morning (pre-bronchodilator),
- After the use of a study drug (post-bronchodilator) from day 30 (steady state) until completion of the double-blind treatment.
Secondary outcome measures
- The rate of decline in the mean forced vital capacity (FVC) and slow vital capacity (SVC).
- HRQoL, measured by the total score on SGRQ, in which scores range from 0 to 100, with lower scores indicating improvement and a change of 4 units or more considered to be clinically meaningful.
- Exacerbations of COPD (as defined below) and related hospitalizations;
- Rate of death from any cause and from lower respiratory conditions.
Patients received either 18 mcg of tiotropium or a matching placebo once daily, delivered through the Handi Haler inhalation device. All respiratory medications, except other inhaled anticholinergic drugs, were permitted during the trial. Smoking cessation programs were offered to all patients before randomization and self-reported smoking behaviour was recorded at each visit. At the end of the study, all patients were provided with and asked to take 40 mcg of ipratropium (two inhaler actuations) four times daily and to return for a final assessment 30 days later.
Patient Selection Criteria
Patients were recruited at 490 investigational centers in 37 countries. Criteria for participation included
- Diagnosis of COPD
- Age of 40 years or more
- Smoking history of at least 10 pack-years,
- Postbronchodilator FEV1, of 70% or less of the predicted value,
- FEV 1 of 70% or less of the FVC (after supervised administration of80 mcg of ipratropium [four actuations], followed by 400 mcg of salbutamol [four actuations] 60 minutes later).
Patients received either 18 mcg of tiotropium or a matching placebo once daily, delivered through the HandiHaler inhalation device
Key exclusion criteria were
- History of asthma
- COPD exacerbation or respiratory infection within 4 weeks before screening;
- History of pulmonary resection;
- Use of supplemental oxygen for more than 12 hours per day;
The presence of a co-existing illness that could preclude participation in the study or interfere with the study results
Procedures
After a screening period, eligible patients were randomly assigned in a 1:1 ratio to receive either tiotropium or placebo. After randomization, clinic visits occurred at 1 month and 3 months and then every 3 months, throughout the 4-year study period.
Spirometry was performed according to the American Thoracic Society guidelines at randomization, at the 1-month visit, at visits every 6 months throughout the study period and at a follow-up visit approximately 30 days after the end of the study.
Eligible patients were randomly assigned in a 1:1 ratio to receive either tiotropium or placebo with the use of centralized randomization in blocks of four, stratified according to site.
HRQoL was measured with the use of the SGRQ before pre-bronchodilator spirometry testing at baseline and every 6 months. Reports of adverse events were collected at each visit.
Exacerbations were defined as an increase in or the new onset of more than one respiratory symptom (cough, sputum, sputum purulence, wheezing, or dyspnoea) lasting 3 days or more and requiring treatment with an antibiotic or a systemic corticosteroid. Data regarding exacerbations and related hospitalizations were collected on study-specific case-report forms at every visit.
Results
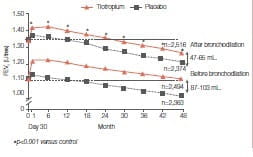
Rate of decline of lung function
In the tiotropium group, the mean values for FVC and FEV1 before and after bronchodilation showed significant improvements in FEV1 compared to the placebo group, ranging from 87 to 103 mL. before bronchodilation and from 47 to 65 mL. afterwards. The rate of decline in subgroups analyses showed no significant differences according to age, gender, smoking status, GOLD stage and reversibility. In a post hoc analysis, between-group differences in the rate of decline in post-bronchodilator FEV1 were observed, with tiotropium again performing better than placebo (40 ± 3 mL. in the tiotropium group and 47 ± 3 mL. in the placebo group, p = 0.046) in the subgroup of 1,554 patients who were not receiving either ICS or LABA at baseline.
There were no significant differences between the study groups in the rate of decline in the mean values for FEV 1 and FVC either before or after bronchodilation from day 30 to the end of study.
The mean FEV value in the tiotropium group after the bronchidialation test showed a significant improvement compared with the placebo group.
The mean FEV1 value in the tiotropium group after the bronchodilatiion test showed a significant improvement compared with the placebo group, which was maintained throughout the study. This improvement ranged from 47 to 65 mL. after bronchodilation (p < 0.001 ). The tiotropium group also showed a significant improvement in the FEV, before bronchodilation, ranging from 87 to 103 mL. (p < 0.001).23 (Figures 19 and 20)
The mean absolute total score on the SGRQ was improved (lower) in the tiotropium group, as compared with the placebo group, at each time point throughout the 4-year period (ranging from 2.3 to 3.3 units, p < 0.001) (Figure 21).
Reduction in exacerbations
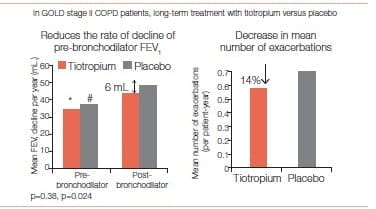
In the UPLIFT study, exacerbations were considered a secondary outcome. Tiotropium was associated with a 14% reduction in the mean number of exacerbation (p>0.001).
The drug was associated with a significant delay in the time to the first hospitalization for an exacerbation (Figure 22).
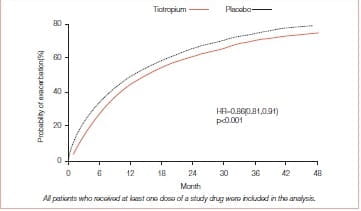
Tiotropium was also associated with a significant delay in the time to the first Exacerbation - a median of 16.7 months (95% Cl: 14.9-17.9) compared with one of 12.5 months (95% Cl: 11.5- 13.8) in the placebo group.
Tiotropium slows disease progression
One analysis of the UPLIFT study focused on the effect of tiotropium versus matching placebo in the 810 (13.5%) COPD patients not on other maintenance treatment (Maintenance (MN)-na’ive: no inhaled LABA, ICS, theophyllines or anticholinergics) at randomization.
The mean absolute total score on the SGRQ was improved (lower) in the tiotropium group, as compared with the placebo group
Means are adjusted for baseline measurements. For FEV1 and FVC, patients with three or more acceptable pulmonary function tests after day 30 and no missing baseline values were included in the analysis. For the SGRQ total score, patients with two or more acceptable scores after month 6 and no missing baseline values were included in the analysis. The I bars represent standard errors, and the horizontal dashed lines represent baseline levels. * p < 0. 001, 1p = 0. 002, and ‡p = 0. 04.
Panel A shows the probability of treatment discontinuation in the tiotropium group and the placebo group (Higher in the placebo group)
Panel B shows the estimated mean forced expiratory volume in 1 second (FEV 1 ) before and after bronchodilation from day 30 to the end of the study
- Before bronchodilation, the annual rates of decline were the same in the tiotropium group and the placebo group: 30 ± 1 mL. per year
- After bronchodilation, the annual rate of decline was 40 ± 1 mL. per year in the tiotropium group, as compared with 42 ± 1 mL. per year in the placebo group
Panel C shows the mean forced vital capacity (FVC) before and after bronchodilation from day 30 to the end of the study
- Before bronchodilation, the annual rate of decline was 43 ± 3 mL. per year in the tiotropium group and 39 ± 3 in the placebo group
- After bronchodilation, the annual rates of decline were the same in the tiotropium group and the placebo group: 61 ± 3 mL. per year
Panel D shows the health related quality-of-life score from month 6 to the end of the study, as measured on SGRQ, which ranges from 0 to 100, with lower scores indicating improvement. The annual rate of change was 1.25 ± 0.09 units per year in the tiotropium group, as compared with 1.21±0.09 units in the placebo group.
Spirometry, HRQoL, exacerbations of COPD and mortality were analysed. 403 patients received tiotropium (age 63 ± 8 years, post-bronchodilator FEV1, 53 ± 12% predicted) and 407 received placebo (age 64 ± 8) years, post bronchodilator FEV 151 ± 12% predicted).
- Post-bronchodilator FEV 1 decline was 42 ± 4 mL. per year in the tiotropium group and 53 ± 4mL. per year in placebo (p=0.026).
- At 48 months, the morning pre-dose FEV1 was 134 mL. higher in the tiotropium group compared to placebo (p < 0.001)
- SGRQ total score declined more slowly in the tiotropium group(difference 1.05 ± 0.34 units per year, p=0.002).
- This was particularly significant for the domains “impact” (difference 1.08 ± 0.37 units per year -1, p=0.004) and “activity” (1.44 ± 0.40 units per year -1, p < 0.001), but not for symptoms (0.26 ± 0.50 units per year -1, p=0.6).
- At 48 months, the SGRQ total score was 4.6 units (p < 0.001) with tiotropium, compared to placebo.
Tiotropium was also associated with a significant delay in the time to the first exacerbation – a median of 16.7 months
In patients with COPD not on maintenance therapy, tiotropium was, therefore, associated with significant benefits in disease progression.
The UPLIFT trial conclusively proved that in patients with COPD, therapy with tiotropium was associated with improvements in the lung function, quality of life, and exacerbations during a 4-year period.
A 9-month, randomised, double-blind, multicentre study19 compared the effect of once-daily tiotropium 18 mcg and placebo on the HRQoL, spirometric parameters and exacerbations in 554 patients with moderate- to-severe COPD. The HRQoL was assessed using the SGRQ and the new 8-item Visual Simplified Respiratory Questionnaire (VSRQ), which is currently being validated.
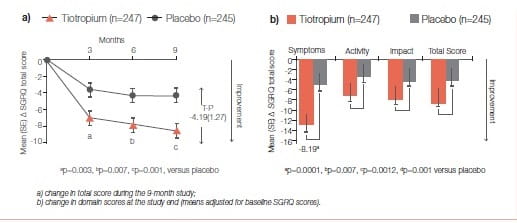
The primary efficacy endpoint was the proportion of patients achieving a reduction of at least 4 units in the SGRQ total score at the study end (month 9). Mean ± SD baseline SGRQ total score was 47.4 ± 18.1 (Figure 23)29.
Significantly more tiotropium-treated patients achieved a reduction of at least 4 units in the SGRQ score versus placebo at study end (59.1% versus 48.2%, respectively; p=0.029).
Tiotropium significantly improved spirometric parameters (FEV): 0.11 ± 0.02 L versus 0.01 ± 0.02 L; between-group difference: 0.10 ± 0.03 L, p=0.0001) and reduced exacerbations versus placebo (Figure 24).
Maintenance treatment with tiotropium provided significant and clinically relevant improvements in HRQoL, as measured by the SGRQ. This has been endorsed in several previous studies.
Tiotropium Lowers Mortality
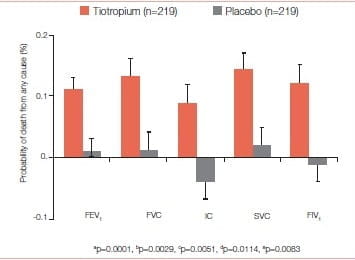
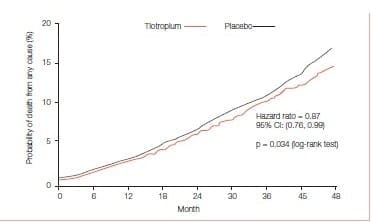
Vital-status information available after a 45-month follow-up for 98% of patients in the tiotropium group and for 97% in the placebo group (including patients who discontinued treatment) showed that tiotropium significantly reduced mortality. Totally 921 patients died-14.4% in the tiotropium group and 16.3% in the placebo group (hazard ratio: 0.87; 95% Cl: 0.76 to 0.99) (Figure 25).
The authors concluded that treatment with tiotropium over 4 years is associated with decreased mortality, with the effect being most prominent in the cardiac and respiratory systems.
Benefits in patients with GOLD stage II disease27
In a sub-analysis of patients with GOLD stage II disease, the rate of decline of the mean post-bronchodilator FEV1 was lower in the tiotropium group than in the control group (43 mL. per year versus 49 mL. per year, p = 0.024).
Treatment with tiotropium over 4 years is associated with decreased mortality, with the effect being most prominent in the cardiac and respiratory systems
For pre-bronchodilator pulmonary function, 1,221 patients in the tiotropium group and 1,158 in the control group had three or more measurements and were included in the analysis. The rate of decline of the mean prebronchodilator FEV1 did not differ between groups (35 mL. per year versus 37 mL. per year; p = 0.38)
Health status, measured with the SGRQ was better at all timepoints in the tiotroplum group than in the control group (p < 0.006 for all timepoints). Time to first exacerbation and time to exacerbation resulting in hospital admission were also longer in the tiotropium group than in the control group (hazard ratio: 0.82, 95% Cl: 0.75- 0.90, and 0.74, 0.62- 0.88, respectively) (Figure 26).
The authors of this sub-analysis concluded that “Tiotropium seemed to reduce the rate of decline of post-bronchodilator FEV in patients with GOLD stage II COPD. This finding and the other improvements in the outcomes suggest that the treatment of COPD should begin at an early stage of the disease.”
Tiotropium seemed to reduce the rate of decline of post bronchodilator FEV1 in patients with GOLD stage II COPD
Among smokers, tiotropium was associated with the following:
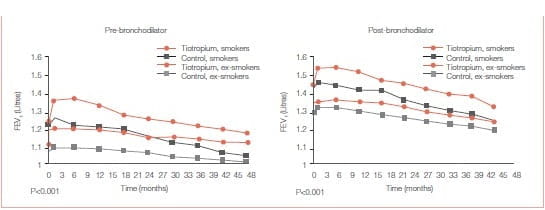
- Significant improvements in pre and post-bronchodilator FEV1 over placebo that persisted throughout the 4-year trial for each smoking status (Figures 27 A & B).
Continuous smokers showed a worse outcome than continuous ex-smokers in terms of the rate of declinein both pre- and post-bronchodilator FEV1 BUT Significant improvements in lung function was observed with tiotropium compared to the control group within each of the three smoking behaviour categories.
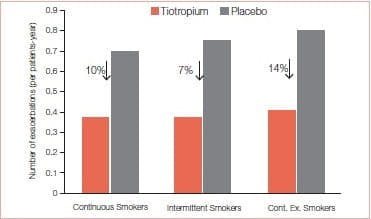
- Significantly reduced exacerbation risk in these patients although differences among categories were observed (Figure 28).
At 4 years, SGRQ for tiotropium patients improved the most in continuous smokers (-4.62 units, p = 0.0006) and the least in intermittent smokers (-0.54 units, p = 0.55), compared with control.
Significant improvements in lung function was observed with tiotropium compared to the control group
Patient Outcomes
The UPLIFT trial conclusively proved that in patients with COPD, therapy with tiotropium was associated with improvements in lung function, quality of life, and exacerbations during a 4-year period. Treatment offered significant benefits in slowing disease progression. Most significantly, treatment with tiotropium over 4 years is associated with decreased mortality, with the effect being most prominent in the cardiac and respiratory systems.
Tiotropium in Asthma
Anticholinergics in Asthma
The role of tiotropium as a treatment for asthma is a subject of recent clinical investigation. Asthma is a chronic inflammatory disorder of the airways that affects 235 million people worldwide and contributes to the overall healthcare burden. However, despite the best-directed treatment, some patients experience frequent exacerbations and have poorly controlled asthma. Poorly controlled asthma affects both the patient and the doctor. Data from the 2011 Asia-Pacific Asthma Insight and Management (AP-AIM) survey said that, in India, zero percentage of asthmatics achieve guideline-defined asthma control despite the use of available medications.
In asthma patients on ICS and LABA therapy, there is a considerable section of the population who experience frequent exacerbations and have persistent airflow limitation. Addition of tiotropium in such patients significantly benefits patients having uncontrolled asthma. Since exacerbations worsen the inflammation and lead to significant airflow limitation, the relevance of the cholinergic system in asthma has gained attention in recent times.
Tiotropium is a long-acting cholinergic antagonist that has been indicated for COPD for over a decade and is now recommended as an alternative add-on treatment option in adults at step 4 and 5 of the GINA guidelines. Also the ICS/NCCP Guidelines 2014 recommend use of tiotropium in step 4 of their recommendation.
Studies of tiotropium in asthma have suggested that tiotropium is a simple and cost-effective alternative as an add-on maintenance bronchodilator treatment in adult patients with asthma who are currently treated with the maintenance combination of ICS (≥800 mcg budesonide/day or equivalent) and LABA and who experienced one or more severe exacerbations in the previous year.
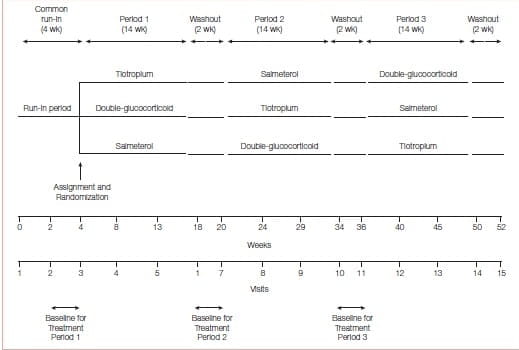
Tiotropium Bromide Therapy for Adults with Uncontrolled Asthma30
This was a three-way, double-blind, triple-dummy crossover trial involving 210 patients with asthma, in which addition of tiotropium (a long-acting anticholinergic agent approved for the treatment of COPD but not asthma) to an inhaled glucocorticoid, as compared with a doubling of the dose of the inhaled glucocorticoid (primary superiority comparison) or the addition of the LABA salmeterol (secondary non- inferiority comparison) was evaluated.
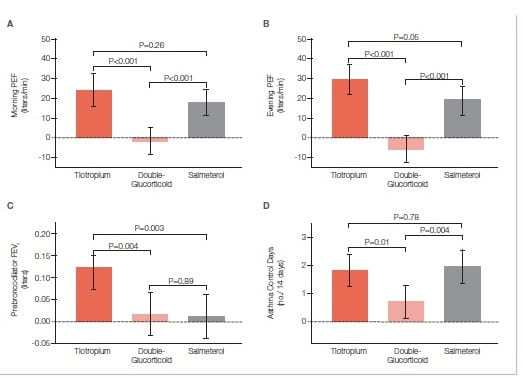
The use of tiotropium resulted in a superior primary outcome (Figure 30), as compared with a doubling of the dose of an inhaled glucocorticoid, as assessed by measuring the morning PEFR, with a mean difference of 25.8 liters per minute (P<0.001) and superiority in most secondary outcomes, including evening PEF, with a difference of 35.3 liters per minute (P<0.001); the proportion of asthma-control days, with a differenceof 0.079 (P=0.01); the FEV1 before bronchodilation, with a difference of 0.10 liters (P=0.004); and daily symptom scores, with a difference of −0.11 points (P<0.001). The addition of tiotropium was also non- inferior to the addition of salmeterol for all assessed outcomes and increased the pre-bronchodilator FEV1 more than did salmeterol, with a difference of 0.11 liters (P=0.003).
When added to an inhaled glucocorticoid, tiotropium improved symptoms and lung function in patients with inadequately controlled asthma. Its effects appeared to be equivalent to those with the addition of salmeterol.
The study shows that the use of tiotropium was superior to a doubling of the dose of an inhaled glucocorticoid for patients whose symptoms were inadequately controlled while they were receiving inhaled corticosteroids. Second, among patients in the study who were similar to those in trials showing the clinical efficacy of LABA therapy tiotropium was non-inferior to salmeterol on the basis of predefined criteria, a finding that meets the standards established in the FDA’s draft guidance for industry on non-inferiority clinical trials.
Tiotropium in Asthma Poorly Controlled with Standard Combination Therapy31
Study Design
Two replicate, randomized, controlled trials assessed the impact of adding tiotropium (a total dose of 5 mcg, n=456) or placebo (n=456) of both delivered by a soft-mist inhaler once daily, on lung function and exacerbations in 912 patients with asthma who were receiving ICS and LABAs, for 48 weeks.
All the patients were symptomatic, had a post-bronchodilator FEV1 of 80% predicted or less and a history of at least one severe exacerbation in the previous year. The patients had a mean baseline FEV of 62% of the predicted value; the mean age was 53 years.
Results
Addition of tiotropium to ICS/LABA provided sustained bronchodilation, had positive outcomes on severe exacerbation and asthma worsening, improved asthma control, and was safe to use in asthma patients.
Primary Endpoints
1) Peak FEV1 (measured 3 hours after treatment) and trough FEV 1 at week 24 expressed as change from baseline
- Significant reduction in airflow obstruction with the addition of tiotropium, as compared to placebo.
At 24 weeks, the mean difference between the tiotropium group and the placebo group in the change in the adjusted peak FEV1 from baseline in the first 3 hours after the administration of tiotropium was86 mL. in trial 1 (P = 0.01) and 154 mL. in trial 2 (P<0.001) (Figure 31)
The change from baseline in the trough FEV in between-group difference at 24 weeks was also significantly greater for patients in the tiotropium group than the placebo group: 88 mL. in trial 1 (P=0.01) and 111 mL. in trial 2 (P<0.001).
2. Severe exacerbation*
- Increased the time to the first severe exacerbation by 56 days when compared with placebo.
- Reduced the risk of a severe exacerbation by 21% (hazard ratio, 0.79; P<0.03).
*Severe exacerbation was defined as asthma necessitating the initiation or doubling of systemic corticosteroid therapy for ≥3 days.
Secondary Endpoints
- Asthma worsening#
- Increased the median time to the first worsening of asthma by 134 days when compared with placebo (hazard ratio, 0.69; P<0.001)
#Asthma worsening was defined as ≥2 consecutive days of progressive increase in one or more asthma symptoms that were outside the patient’s usual range of day-to-day asthma symptoms, or a decreased best morning PEFR of ≥30% from baseline morning PEFR.
- Improved asthma control
- Increased the Asthma Quality of Life Questionnaire (AQLQ) and Asthma Control Questionnaire 7 (ACQ-7##) total scores, though it did not reach the minimal clinical important difference (MCID). However, tiotropium showed a greater likelihood of experiencing a MCID in ACQ-7 (58.1% tiotropium versus 45.1% placebo). This suggests that there is improvement in symptom control in patients in the tiotropium group.
##ACQ-7 Score ranges from 0 (no impairment) to 6 (maximum impairment). A patient was considered to be a responder if there was an improvement (decrease) in the ACQ total score of at least 0.5 points.
- Safety
- No deaths occurred. Similar rate of adverse events were observed in the tiotropium and placebo groups
- Dry mouth was reported by 11 patients: 8 (1.8%) in the tiotropium group and 3 (0.7%) in the placebo group. Only allergic rhinitis occurred at a significantly higher rate in the tiotropium group.
Conclusion
In patients with poorly controlled asthma despite treatment with ICS and LABAs, the use of tiotropium as an add-on therapy significantly increased the time to the first severe exacerbation, reduced the risk of asthma worsening and provided sustained bronchodilation.
Tiotropium and ACOS
Simplifying ACOS
Asthma and COPD are distinct clinical entities. Asthma COPD overlap syndrome (ACOS) is a condition wherein the patient presents some features of asthma and some of COPD at the same time. Prevalence of ACOS among adult patients with COPD or asthma ranged from 12 to 55%.32 ACOS patients have more severe disease, with increased exacerbations and hospitalizations, compared with some asthma and COPD patients.33 Tiotropium might prove useful in ACOS patients who need an ICS/LABA combination with the additional bronchodilation of a LAMA or in patients with severe asthma when a LAMA might provide additional bronchodilation and asthma control.34
Improvements with Tiotropium in COPD patients with concomitant asthma35
COPD and asthma have had different diagnostic criteria and treatment paradigms. Both have many common overlapping parameters and might occur in the same patient.
In this 12-week randomized, double-blind, placebo-controlled, parallel-group study with tiotropium 18 mcg (monotherapy), the spirometric effects of tiotropium in COPD patients (N=472, FEV =1.55 L, 53.0% predicted) with concomitant asthma were determined.Inclusion Criteria
Physician diagnosis of COPD and asthma, age ≥40 years, smoking >10 pack-years, post-bronchodilator FEV<80% predicted, FEV /FVC <70%, ≥12% and ≥200 mL. increase in FEV following inhaled bronchodilator, and treatment with ICS ≥1 year. Spirometry was measured serially for 6 hours on days 1, 29 and 85.
- Improvements at 12 weeks with triotropium were observed for the primary endpoint FEV1 area under the curve (AUC) from 0–6 hours (difference = 186±24 mL., p<0.001) and for morning pre-dose FEV (difference = 98±23 mL., p<0.001).
- Significant differences in favor of tiotropium were observed for pre-dose FVC (difference = 128±34 mL., p<0.001) and FVC AUC 0–6 hours (difference = 232±35 mL., p<0.001).
- Compared to baseline, the mean weekly number of daily puffs of salbutamol was reduced by 0.05±0.12 puffs/day in the placebo group and by 0.50±0.12 puffs/day in the tiotropium group at week 12 (p<0.05).
- Tiotropium showed improvements in lung function along with symptomatic benefit as seen with a reduced need for rescue medication in patients with COPD and concomitant asthma.
Safety and Efficacy of Tiotropium
COPD is a common cause of hospitalizations and is a rapidly increasing cause of mortality worldwide. Despite the heavy burden of COPD- related illness, the leading cause of hospitalization in COPD patients is cardiovascular disease. This link between COPD and cardiovascular disease is in part due to the fact that both diseases share common risk factors, such as tobacco smoking and advanced age. It is also hypothesized that systemic inflammation in COPD increases the risk for cardiac events such as myocardial infarction.
Tiotropium was associated with a decreased risk for a cardiac adverse event and no increased risk for a vascular adverse event.
There has been conflicting evidence concerning cardiovascular risk associated with inhaled anticholinergics agents used for the treatment of COPD. In this context, it is important to discuss the cardiovascular safety of tiotropium.
Adverse Events
Pooled data revealed, 1,511 patients with at least one cardiac adverse event and 1,233 patients with at least one vascular adverse event (Table 3). The Incidence rate difference (RD) (95% Cl) for any cardiac event (tiotropium minus placebo) was -0.79 (-1.48, -0.09) and for any vascular event was -0.14 (-0.77, 0.49).
Tiotropium was associated with a decreased risk for a cardiac adverse event and no increased risk for a vascular adverse event. The absence of increased risk was observed for the most common events in the categories of hypertension, ischemic heart disease and cardiac failure.
Cardiac failure occurred at a decreased rate with tiotropium relative to placebo (RD (95% Cl) = -0.47 (-0.84, -0.1 0). For myocardial infarction and stroke, the RDs (95% Cl) were -0.18 (-0.42, 0.05) and 0.04 (-0.20, 0.27), respectively. The incidence rates for stroke were similar between the two treatment arms.
|
|
IR (Placebo) |
IR (Tiotropium) |
RD (Tiotropium-Placebo) |
|
Cardiac disorders |
7.26 |
6.47 |
−0.79 * |
|
Atrial fibrillation/flutter |
1.27 |
1.14 |
−0.12 |
|
Cardiac arrest |
0.28 |
0.17 |
−0.09 |
|
Cardiac failure |
2.32 |
1.83 |
−0.47* |
|
Ischemic heart disease |
2.56 |
2.29 |
−0.28 |
|
Myocardial infarction |
0.94 |
0.75 |
−0.18 |
|
Palpitations |
0.48 |
0.53 |
0.04 |
|
Supraventricular tachycardia |
0.22 |
0.25 |
0.03 |
|
Tachycardia (nonventricular) |
0.44 |
0.48 |
0.02 |
|
Ventricular tachycardia/fibrillation |
0.24 |
0.15 |
−0.09 |
|
Vascular disorders |
5.68 |
5.50 |
−0.14 |
|
Aneurysm |
0.36 |
0.40 |
0.05 |
|
Hypertension |
3.47 |
3.13 |
−0.34 |
|
Stroke |
0.85 |
0.87 |
0.04 |
|
Lower respiratory disorders |
65.02 |
53.16 |
−14.2* |
|
COPD exacerbation |
43.18 |
34.87 |
−8.90* |
|
Pneumonia |
4.96 |
4.78 |
−0.25 |
|
Respiratory failure |
1.89 |
1.46 |
−0.38* |
Abbreviations: COPD, chronic obstructive pulmonary disease; IR, incidence rate (per 100 patient years of time at risk). Incidence rate (IR) =number of patients with an event/cumulative time at risk within a treatment group.
Incidence rate difference (RD) = IR (tiotropium) -IR (placebo).
Incidence rate differences were estimated based on the method described by Greenland and Robins.
The 95% confidence interval (CI) was calculated for each rate difference in order to describe the precision of the effect estimate. A RD < 0 indicates a decreased risk with tiotropium and a RD > 0 indicates a decreased risk with placebo.
* P< 0.05. P value less than 0.05 is indicated when the width of the CI excludes the value 0.
. (Kesten S. Tiotropium HandiHaler® in the treatment ofCOPD: A safety review. Int J Chron Obstruct Pulmon Dis. 2009; 4: 397-409)
There was no indication of an increased risk for myocardial infarction or stroke associated with tiotropium.
Lower respiratory tract events were common and occurred with a lower rate in the tiotropium group (RDs,-14.2; 95% Cl: -17.0, -11.5). The most common event was reported as a COPD exacerbation, which along with respiratory failure, occurred with a tiotropium minus placebo rate difference and the upper limit of the Cl below zero.
In the UPLIFT study, at 1,440 days with 95% of patient outcome accounted for, tiotropium was associated with a significant 13% reduction in all-cause mortality compared to placebo. However, at 1,470 days with only 75% of patient outcome accounted for, tiotropium was associated with a non-significant 11% reduction in all-cause mortality compared to placebo. The relative risks for serious cardiovascular events, heart failure, and myocardial infarction were all significantly lower with tiotropium than placebo (Figure 35)
There was a lower incidence rate for a major cardiovascular event in the tiotropium group relative to the placebo group (-0.45 patients per 1 00 patient years at risk) and a lower rate for fatal cardiovascular events (-0.32 patients per 100 patient-years at risk).
There was a lower incidence rate for a fatal event during treatment in the tiotropium group relative to the placebo group, with the upper limit of the Cl being less than zero.
The relative risks for serious cardiovascular events, heart failure, and myocardial infarction were all significantly lower with tiotropium than placebo.
Dosing equivalence
Tiotropium delivered at a dose of 5 μg has showed efficacy similar to that of 18 μg of tiotropium in placebo- controlled trials involving patients with COPD. TIOSPIR was a randomized, double-blind, parallel-group trial.36 This trial evaluated the safety and efficacy of tiotropium in 17,135 COPD patients at a once daily dose of 2.5 μg (N=5724) or 5 μg (N=5705), compared with that of tiotropium at a once daily dose of 18 μg (N=5687). Primary end points were the risk of death (non-inferiority study, Tiotropium 5 μg or 2.5 μg vs. Tiotropium 18 μg) and the risk of the first COPD exacerbation (superiority study, Tiotropium 5 μg vs. Tiotropium 18 μg)
During a mean follow-up of 2.3 years, Tiotropium at a dose of 5 μg or 2.5 μg was non-inferior to Tiotropium 18 μg with respect to the risk of death (Tiotropium 5 μg vs. Tiotropium 18 μg: HR, 0.96; Tiotropium 2.5 μg vs. Tiotropium 18 μg: HR, 1.00) and not superior to Tiotropium 18 μg with respect to the risk of the first exacerbation (Tiotropium 5 μg vs. Tiotropium 18 μg: HR, 0.98). Tiotropium at a dose of 5 μg or 2.5 μg had a safety profile and exacerbation efficacy similar to that of tiotropium 18 μg in patients with COPD.
Conclusion
Recently, there has been a drastic change in the manner in which the role of tiotropium has been viewed. Tiotropium has been conveniently positioned for COPD and now has also repositioned itself as an add-on to ICS/LABA in uncontrolled asthma. A better understanding of these diseases has aided better treatment.
In sync with this better understanding, there has been a plethora of newer updates on tiotropium use in COPD and asthma management.
Tiotropium has been demonstrated to be effective in COPD treatment, with various research publications in improvements in lung function, dynamic hyperinflation, pulmonary rehabilitation, exercise capacity, walking endurance, night-time awakening, and rescue medication use.
In addition, tiotropium has also proved its role in exacerbations of COPD by prolonging time to first exacerbation, increasing time to first hospitalization, and displaying a protective role in case of moderate exacerbations in patients at risk of frequent exacerbation.
Tiotropium has been compared with ICS/LABA and also in combination with formoterol in the treatment of COPD. Combination therapy is effective in reducing rescue medication use, and enabling improvement in dyspnea, lung function and health status.
In the overall assessment of safety and efficacy, tiotropium is considered as an invaluable option in COPD management and it continues to exhibit a highly favorable benefit profile in COPD treatment. It has now found its way in treating uncontrolled severe asthma, with a safety profile similar to placebo.
Tiotropium has also substantiated its role in COPD patients with concomitant asthma with improvements in lung function along with a reduced need for rescue medication.
References
1. http://www.ncbi.nlm.nih.gov/pubmed search tiotropium+COPD, Accessed on 25/05/2016
2. Int J Chron Obstruct Pulmon Dis 2007:2(1) 33-40
3. Int J Chron Obstruct Pulmon Dis 2006:1(2) J07-J14.
4. Chest. 2000; 117(2 Suppl): 63S-6S
5. Proc (Bayl Univ Med Cent). 2004, 17(3):366-73.
6. Chest 2008; 133; 1451-1462.
7. COPD: Journal of Chronic Obstructive Pulmonary Disease, 6:3, 211- 225
8. Eur Respir J 2004; 23: 832--840.
9. Chest 2005; 128;1168-1178.
10. Eur Respir J 2012; 39, 265–271 doi: 10.1183/09031936.00059511
11. Lung. 2006; 184(4): 201-4.
12. Chest. 2005; 127(3):809-17
13. Int J Chron Obstruct Pulmon Dis. 2008;3: 127-36.
14. Eur RespirJ 2002; 19:217-224.
15. Eur Respir J 2002; 19(2): 209-16.
16. The Open Respiratory Medicine Journal, 2009; 3, 43-52
17. Ann Am Thorac Soc 2014; 11: 9, 1351–1361 doi: 10.1513/AnnalsATS.201404-174OC
18. Respir. Res. 2016; 17:27 doi: 10.1186/s12931-016-0340-9
19. Respir. Med. 2016; 114, 1-8
20. Respir. Med. 2010; 104 (10): 1460-1472
21. N. Engl. J. Med. 2008; 359 (15) 1543-1554.
22. Ann. Intern. Med. 2005; 143 (5) 317-326.
23. Pharmacoepidemiol Drug Saf. 2016, 25: 5, 1099-1557 doi:10.1002/pds.3961
24. Eur Respir J. 2010;35(2): 287-94.
25. COPD. 2004, 1(2):303-1 2
26. N Engl J Med. 2008;359(I5): 1543-54.
27. Eur Resp11·J. 2010;36(I):65- 73.
28. Lancet.2009, 3 74(9696): I 171-8.
29. Int J Chron Obstruct Pulmon Dis 2008;3(2):301-10.
30. N Engl J Med. 2010; 363(18): 1715–1726. doi:10.1056/NEJMoa1008770
31. N Engl J Med 2012. doi: 10.1056/NEJMoa1208606
32. Expert Rev Respir Med. , 10:3, 363-371, DOI: 10.1586/17476348.2016.1144476
33. Prim Care Respir Med 2015; 25, 15047; doi:10.1038/npjpcrm.2015.47
34. J Allergy Clin Immunol 2015;136:531-45
35. Respi. Med. 2008; 102, 50–56
36. N Engl J Med 2013;369:1491-501
37. Chest 2010;137(1):20-30