The Sunshine Vitamin and Infants: Exploring the Need
Introduction to Vitamin D
Till a few decades ago, vitamin D was only related to bone health and calcium homeostasis. Now, medical and nonmedical fraternities across the world are getting increasingly curious and realizing the potential role of vitamin D in health and disease.1
Synthesis
Vitamin D is a fat-soluble vitamin that functions as a hormone in the body. It is a steroid hormone acting on specific cell receptor to regulate various tissue processes. Main forms of vitamin D in nature are vitamin D2 (ergocalciferol), which is photochemically synthesized in plants, and vitamin D3 (cholecalciferol), which is synthesized in the skin of animals and humans in response to sunlight.
The synthetic pathway involves 25- and 1-alpha-hydroxylation of vitamin D2 and D3, in the liver and kidneys, respectively. First, hydroxylation occurs within the liver and leads to the formation of 25(OH)D or calcidiol; secondly, hydroxylation occurs within the kidneys and constitutes the most biologically active hormonal form of vitamin D: 1,25(OH)2D, or calcitriol. Calcitriol, the active vitamin D form, binds to the vitamin D receptor (VDR) to
- increase intestinal calcium and phosphate absorption;
- increase bone resorption;
- decrease renal calcium and phosphate excretion; and
- exhibit metabolic, immune and extra-skeletal effects.1
Sources
Vitamin D is primarily made in the skin (>90%) after exposure to ultraviolet radiation (UVR), and <10% is derived from dietary sources (as vitamin D is the precursor of a steroid hormone and not a nutrient by design). Natural food sources of vitamin D include the following:
- Oily fish such as salmon, mackerel, sardines, tuna, hilsa and cod fish, cod liver oil, and
- Liver and organ meats3
Role
- Vitamin D has a wide spectrum of actions, ranging from its role in bone health and calcium homeostasis to extraskeletal effects on the immune system and cell proliferation.
- Vitamin D is required to maintain normal blood levels of calcium and phosphate, which, in turn, is needed for the normal mineralization of bone.
- Vitamin D may also play a role in muscle contraction, nerve conduction and general cellular function in all cells of the body.
- The immunomodulatory properties may explain the reported associations between vitamin D deficiency on one hand and metabolic diseases such as type 2 diabetes, autoimmune diseases, infections such as tuberculosis, and some malignancies on the other.4
Measurement of Vitamin D Status
25(OH)D is the major circulating form of vitamin D. It has a half-life of 2-3 weeks and its levels are the best available indicators of vitamin D status. Although 1, 25(OH)2D (calcitriol) is the active form, it has a half-life of only 4 hours and it is not a good indicator of vitamin D stores because
(i) vitamin D deficiency can cause parathyroid hormone (PTH) elevation that induces increased 1-alpha hydroxylase activity, which results in normal or increased levels of 1,25(OH)2D; and
(ii) it circulates at a concentration that is 100- to 1,000-fold less than 25(OH)D.
The assays used to measure 25(OH)D levels should be capable of measuring both D2 (ergocalciferol) and D3 (cholecalciferol) derivatives. There is debate centreed upon what constitutes normal levels of 25(OH)D. It depends on the level at which (a) PTH remains in the normal range; (b) bone resorption is minimal; and (c) calcium absorption is maximally enhanced. Many study authors have found this level to be 20 ng/mL (50 nmol/L).
Table 1: Vitamin D Status in Relation to 25(OH)D
|
Vitamin D status |
Levels |
|
US Endocrine Society Classification |
|
|
Sufficiency |
>20 ng/ml |
|
Deficiency |
<15 ng/ml |
|
Severe deficiency |
<5 ng/ml |
|
Risk of toxicity |
>50 ng/ml |
|
US Institute of Medicine Classification |
|
|
Sufficiency |
>30 ng/ml |
|
Insufficiency |
21-29 ng/ml |
|
Deficiency |
<20 ng/ml |
|
Toxicity |
>150 ng/ml |
1 mcg = 40 I.U.; 0.025 mcg is 1 I.U.
Serum levels of 25(OH)D <20 ng/dL are defined as vitamin D deficiency, whereas 21–29 ng/dL is considered to be insufficient by the US Endocrine Society. It has been estimated serum 25(OH)D levels of 20 ng/dL meet the needs of at least 97.5% of the population across all age groups in developed countries. Hence, it has been concluded by the Institute of Medicine that 25(OH)D levels >20 ng/dL indicates vitamin D sufficiency.2
It has been estimated that 1 billion people worldwide have vitamin D deficiency or insufficiency. Though the majority of the population in India lives in areas receiving ample sunlight throughout the year, vitamin D deficiency is very common in all the age groups and both the sexes across the country. The prevalence of deficiency (as defined by 25(OH)D <20 ng/mL in most of the studies) ranges from 75% to 90%.
There are several factors that place individuals at risk for vitamin D deficiency. Infants who are breastfed appear to be at higher risk of vitamin D deficiency.
Vitamin D and Infants
Vitamin D deficiency along with a resurgence of rickets is increasingly being reported in young infants from temperate regions, African-Americans, and also from India (~ 30%).5 Recent data indicate that vitamin D deficiency is pandemic and even the healthy and the young are not spared.1
Vitamin D deficiency in neonates and infants can lead to serious consequences such as hypocalcaemic seizures and increased risk of respiratory tract infections.3 Rickets is an extreme form of vitamin D deficiency and represents the tip of the vitamin D deficiency iceberg.1
Demography of Vitamin D Deficiency in Infants in India
Till the early 1990s, vitamin D deficiency was considered to be rare in India.6 In spite of being a sunlight-rich country, India has demonstrated widespread vitamin D deficiency across all age groups.3
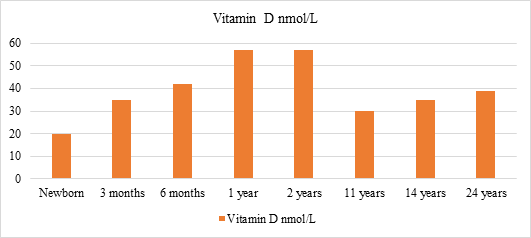
Fig. 1: Vitamin D concentrations as reported by various Indian studies
A high prevalence of severe vitamin D deficiency (25(OH)D levels <10 ng/mL) was recorded among study infants with hypocalcaemic seizures (90%), and control infants (41.7%) in a hospital-based study. Recently, a study from southern India reported rickets and hypocalcaemic seizures due to vitamin D deficiency in exclusively breastfed young infants.6
Infants - The Vulnerable Group
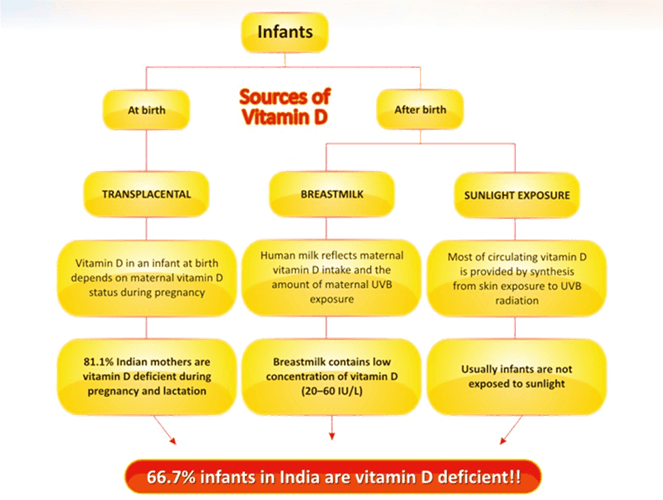
Vitamin D sources in early infancy consist of transplacental stores, human milk, and cutaneous production via sunlight.
Fig 2: Causes of vitamin D deficiency in infants
At Birth
Previous placental transfer: Maternal vitamin D and 25(OH)D cross the placenta during the last months of gestation and establish vitamin D stores for the newborn. Studies have found that 81.1% of Indian mothers are vitamin D-deficient. Decreased vitamin D in the mother results in decreased transplacental transfer of vitamin D and, thus, reduced stores at birth.7 A recent study has shown that maternal vitamin D deficiency can also lead to a three times increased risk of vitamin D deficiency in infants.8The serum 25(OH) vitamin D levels in the cord blood have an excellent, positive correlation with the mother’s serum vitamin D levels soon after delivery, which persists till 12–16 weeks of age. After this, infants have to rely on other sources of vitamin D, in the absence of which, the infant becomes vitamin D-deficient.7
After Birth
Human milk: After birth, the source of vitamin D for an infant depends on the intake from human milk, mostly as parent vitamin D and 25(OH)D. The milk of healthy lactating women contains relatively small amounts of vitamin D (20–60 I.U./L). Vitamin D in breast milk relates to the mother’s vitamin D intake, skin pigmentation and sunlight exposure. Assuming an average consumption of 750 mL/day, exclusive breastfeeding without sun exposure would provide only 11–38 IU/day of vitamin D, which is far below the recommended minimum intake.7 This implies that babies born to mothers with vitamin D deficiency are very likely to develop vitamin D deficiency unless supplemented from outside or adequately exposed to sunlight, which is often not practical during early infancy.8
Sunlight exposure: Most of the circulating vitamin D is provided by synthesis from skin exposure to UVB radiation (>90%). However, to maintain vitamin D level in the sufficiency range, the duration of UVR exposure, particularly in relation to time of the day, season or skin pigmentation, remains to be determined. The traditional custom of giving infants an oil massage in the sunlight for 15–30 minutes before bathing has gradually declined, especially in the cities. Moreover, the intake of vitamin D is inadequate as food items (except infant formulas) are not fortified.8
Maternal Vitamin D Status and Long-term Effects on the Infant8
Studies have shown a strong relationship between maternal and foetal circulating (cord blood) 25(OH)D concentrations. An unsupplemented infant born to a vitamin D-deficient mother will reach a state of deficiency more quickly than an infant whose mother was replete during pregnancy. Adequate nutritional vitamin D status during pregnancy is important for foetal skeletal development, tooth enamel formation and, perhaps, general foetal growth and development. There is some evidence that the vitamin D status of the mother has long-term effects on her infant.A recent Canadian study by Mannion et al. compared growth parameters in newborn infants with the maternal intakes of milk and vitamin D during pregnancy. Investigators found an association between vitamin D intake during pregnancy and birth weight but not infant head circumference or length at birth. With every additional 40 IU of maternal vitamin D intake, there was an associated 11 g increase in birth weight.
Another study of the intrauterine effect of maternal vitamin D status revealed a significant association between umbilical cord 25(OH)D concentrations and head circumference at 3 and 6 months’ postnatal age, which persisted after adjustment for confounding factors. A study performed in the United Kingdom during the 1990s demonstrated that higher maternal vitamin D status during pregnancy was associated with improved bone mineral content and bone mass in children at 9 years of age.
Thus, intake of vitamin supplements by an infant, sunlight exposure and maternal 25(OH)D levels were found to have positive correlation with the infant’s 25(OH)D. This suggests that either or all of these three strategies should be effective in raising the infant’s 25(OH)D levels.
Prevention and Treatment of Vitamin D Deficiency
Vitamin D deficiency is ubiquitous, but the estimation of serum 25(OH)D is too prohibitively costly to be recommended as a necessity before preventive supplementation in India.3Therefore, daily supplementation of vitamin D appears to be the most efficient strategy to establish adequate vitamin D status and prevent rickets in infancy.9
Prevention of Vitamin D Deficiency
The recommended vitamin D intake is 400 IU/day in infants less than 1 year of age and 600 IU/day in children more than 1 year of age, as suggested by the US IOM.2 Also, the current recommendation of the American Academy of Pediatrics is that all infants and children, including adolescents, should have a minimum daily intake of 400 IU of vitamin D beginning soon after birth. The Australasian Pediatric Endocrine Group (APEG) also endorses the same recommendation. However, to keep the serum levels of 25(OH)D >30 ng/dL which is considered to be the optimal level, the US Endocrine Committee has suggested the intake of 400–1,000 IU/day under 1 year of age and 600–1,000 IU/day from 1 to 18 years of age.1,2
In their recently revised guidelines, the Indian Council for Medical Research (ICMR) and the National Institute of Nutrition (NIN) do not mention routine use of vitamin D in their RDA, explaining that there is a need to improve education and awareness regarding adequate sunshine exposure. Instead, they leave it to the discretion of the paediatrician or physician, to prescribe 400 IU daily in “situations of minimal exposure to sunlight”.
Multiple studies have shown that daily supplementation of 400 IU in infants is safe and efficacious in raising 25(OH)D levels to sufficiency status. Atas et al. from Turkey compared supplementation of 200 IU versus 400 IU daily and found that all the infants in the 400 IU group had 25(OH)D levels >30 ng/mL.1,2
Table 2: Recommendations of expert groups regarding daily intake and maintenance doses of vitamin D in childhood3
|
Age |
AAP |
IOM (NIH) |
APEG |
Endocrine Society (USA) |
|
< 1 year |
400 I.U. |
400 I.U. |
400 I.U. |
400 – 1000 I.U. |
|
1 – 18 years |
400 I.U. |
400 I.U. |
400 I.U. |
600 – 1000 I.U. |
AAP – American Academy of Pediatrics; IOM – Institute of Medicine; APEG – Australasian Pediatric Endocrine Group
Although vitamin D deficiency is common in exclusively breastfed infants, it may also occur in formula-fed infants. Since most of the infant formulas contain 400 IU/L, infants who are on formula feeds also need supplementation (400 IU/day) unless they consume more than 1,000 mL of formula per day.2
Preterm infants should be supplemented from birth with 400–800 IU/day because of inadequate transfer of maternal vitamin D stores and issues associated with prematurity such as poor feeding, gastrointestinal difficulties impairing absorption and sometimes liver and kidney impairment.2
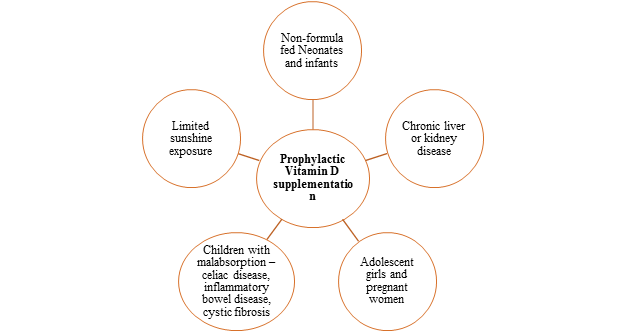
Finally, there are some other vulnerable groups who may require prophylactic vitamin D supplementation. They are as below:
Fig.3: Indications for prophylactic vitamin D supplementation3
Treatment of Vitamin D Deficiency
When to Treat?
Vitamin D therapy is necessary for infants and children who manifest clinical features of hypocalcaemia as a result of vitamin D deficiency or rickets and when vitamin D levels are in the deficient range even if asymptomatic.2
Table 3: Treatment regimens for vitamin D deficiency2
|
Group |
Daily Regimen (8-12 weeks) |
Weekly regimen (8-12 weeks) |
Stoss therapy (Oral or IM) |
Maintenance |
|
< 1 month old |
1000 I.U. |
50000 I.U. |
- |
400 – 1000 I.U. |
|
1 – 12 month old |
1000 – 1500 I.U. |
50000 I.U |
1 lakh – 6 lakhs units over 1-5 days |
400 – 1000 I.U. |
|
1 – 18 years old |
5000 I.U. |
50000 I.U |
3 lakhs – 6 lakhs units over 1-5 days |
600 – 1000 I.U. |
|
>18 years |
6000 I.U. |
50000 I.U |
3 lakhs – 6 lakhs units over 1-5 days |
1500 – 2000 I.U. |
Several therapeutic regimens have been attempted for deficiency of vitamin D. A reasonable approach is as follows:
- For infants aged <1 month: 1,000 IU daily for 3 months and then maintenance with 400 IU daily.
In terms of the Stoss dose preparations, infants may be given 50,000 units stat, which may be repeated if required. However, stoss therapy in not recommended in children <3 months of age.
- For infants aged 1–12 months: 1,000–5000 IU daily for 3 months and then maintenance with 400 IU daily.
In terms of the stoss dose preparations, infants may be given 50,000 units stat weekly for 8–12 weeks or a single I.M. injection of 100,000–600,000 IU over 1–5 days.
- For children aged >1 year: 5,000 IU daily may be given for 3 months followed by maintenance with 600–1,000 IU daily.
In terms of the stoss dose preparations, children may be given 50,000 units stat weekly for 8–12 weeks or a single I.M. injection of 300,000–600,000 IU over 1–5 days.
Short-term administration of vitamin D2 or D3 2,000 units daily or vitamin D2 50,000 units weekly has yielded equivalent outcomes in the treatment of hypovitaminosis D in young children. The total dose of vitamin D has been reported to be more predictive of vitamin D sufficiency rather than the frequency of dosing (daily, weekly or monthly). Therefore, treatment regimens for a given patient can be individualized to ensure compliance, since no difference in the efficacy or safety was reported in these common treatment regimens.
Lack of compliance is an important cause for lack of response to therapy and an option to prevent this is to administer a high dose of 100,000–600,000 IU over 1–5 days (stoss therapy). Doses of 10,000 units/kg instead of smaller doses over a longer period, followed bya maintenance dose, have also been reported to be effective. Another advantage of stoss therapy is that the vitamin D is efficiently stored in adipose tissue and muscle and is continuously converted into its active form. Stoss therapy regimens with large oral or parenteral doses of vitamin D3 have been shown to cause increased and sustained higher levels of 25(OH)D levels, especially the regimen with 600,000 IU. Stoss therapy is safe and can lead to hypercalcaemia only at very high doses. Doses of 150,000–300,000 IU can be effective, with less side effects. After the completion of treatment, vitamin D has to be continued at 800–1,000 IU/day till serum alkaline phosphatase returns to normal, followed by the RDA as per age.2,3High-dose vitamin D may need to be intermittently repeated (usually every 3 months), if poor compliance persists, with maintenance dosing.7
Even for children who are not hypocalcaemic, calcium supplements are important for avoiding subsequent hypocalcaemia from a decrease in bone demineralization and an increase in bone mineralization as PTH levels normalize (hungry bone syndrome), particularly with stoss therapy. Supplementation of elemental calcium in a dose of 30–75 mg/kg/day in three divided doses is recommended. High doses of calcium are necessary early in the course of therapy, after which doses are reduced by half for the next 1–2 weeks.
Monitoring therapy
It is important to obtain calcium, phosphorus and serum alkaline phosphatase (ALP) levels 1 month after initiating therapy. With stoss therapy, the biochemical response is usually evident in 1 or 2 weeks. Usually, calcium and phosphorus levels become normal within 6–10 days, whereas PTH and 25(OH)D levels normalize within 1–2 months and serum alkaline phosphatase by 3–6 months. Complete radiological healing takes longer than 1 month although evidence of healing is seen within 4 weeks.
After 3 months, it is recommended to obtain serum levels of calcium, phosphorus, magnesium, serum alkaline phosphatase, 25(OH)D and PTH, and a repeat X-ray if there are bone changes initially. Subsequently, 25(OH)D levels may be monitored yearly. Considering the cost of performing laboratory tests, reserving investigations only for those not improving (based on clinical assessment) may be an appropriate practical option.2)
Vitamin D toxicity is defined as serum 25(OH)D >150 ng/mL and hypervitaminosis D as 25(OH)D >100 ng/mL. Caution needs to be exercised when treating young children as the administration of dosages to infants that are often used in older children and adults has toxic potential. Hypervitaminosis D can present with serious consequences such as hypercalcaemia, hypercalciuria, concentration defects, renal calculi and even acute renal shutdown. Therefore, emphasis should be laid on using minimum treatment doses of vitamin D that can produce adequate therapeutic response.3
References
1. Narendra Rathi, Akanksha Rathi. Vitamin D and Child Health in the 21st Century, Indian Pediatrics 2011; 48:619–625
2. S. Balasubramanian, K. Dhanalakshmi and Sumanth Amperayani. Vitamin D Deficiency in Childhood – A Review of Current Guidelines on Diagnosis and Management. Indian Pediatrics 2013; 50:669–675
3. Kriti Joshi, Vijayalakshmi Bhatia. Vitamin D Deficiency in a Tropical Country - Treatment and Prevention in Children.Indian J Pediatr 2014; 81(1):84–89
4. CV Harinarayan and Shashank R Joshi. Vitamin D Status in India – Its Implications and Remedial Measures. JAPI 2009;57:40-48
5. S. Balasubramanian, R. Ganesh. Vitamin D deficiency in exclusively breast-fed infants. Indian J Med Res 2008:250–255
6. Urvashi Mehlawat, Priyanka Singh, Shubhra Pande. Current status of Vitamin-D deficiency in India. IPP 2014; 2 (2):328–335
7. Madhusmita Misra, Danie`le Pacaud, Anna Petryk et al. Vitamin D Deficiency in Children and Its Management: Review of Current Knowledge and Recommendations. Pediatrics 2008; 122:398–417
8. Vandana Jain, Nandita Gupta, Mani Kalaivani et al. Vitamin D deficiency in healthy breastfed term infants at 3 months & their mothers in India: Seasonal variation & determinants. Indian J Med Res. 2011;133(3):267–273
9. Gul Yesiltepe Mutlu, Yusuf Kusdal, Elif Ozsu et al. Prevention of Vitamin D deficiency in infancy: Daily 400 IU vitamin D is sufficient, International Journal of Pediatric Endocrinology 2011: