Evidence accumulated from phase-III studies suggests that first-line therapy with imatinib improves response and survival rates in newly diagnosed Philadelphia chromosome positive CML.
In IRIS study, the efficacy of imatinib (400 mg once daily; n=553) was compared with IFN-? (target dose 5 million U/m2/day; n=553) plus low-dose cytarabine (20 mg/m2/day; for 10 days cycle) in the treatment of newly diagnosed chronic-phase CML.1
Current evidence suggests that early response to imatinib treatment has a low overall risk of progression to accelerated phase or blast crisis CML. Therefore, imatinib is recommended as first-line therapy for chronic phase CML.
The scientific basis for imatinib as the standard of care in chronic-phase CML comes from IRIS trial wherein it showed excellent response rates even when used second-line. In this large Phase III, randomized clinical trial, 1106 patients received either imatinib (400 mg once daily; n=553) or IFN-? (target dose 5 million U/m2/day; n=553) plus low-dose cytarabine (20 mg/m2/day; for 10 days cycle) in newly diagnosed chronic-phase CML. Crossover of patients to the other treatment group was allowed if there was no response, loss of response, rise in white blood cell count or intolerance. In this study, a very high rate
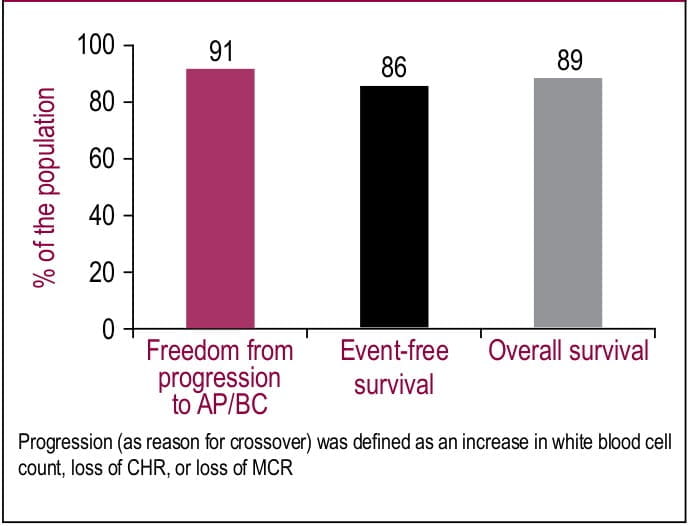
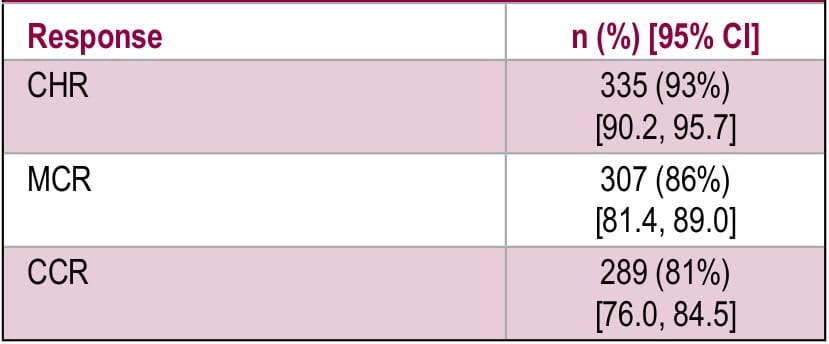
(65%; n=359) of crossover to imatinib arm was observed. In this population, 93% achieved CHR, 86% achieved MCR, and 81% achieved a CCR after a median follow up of 54 months (Table 1). After 4-year follow-up, significant number of patients remitted from progressing to accelerated phase or blast crisis CML (91%; Figure 1). Second-line treatment with imatinib resulted in high rates of event-free survival (86%) and overall survival (89%; Figure 1).
The study suggests that imatinib as a second- line treatment for chronic phase CML provides excellent clinical responses and high survival rates.
Although imatinib has excellent efficacy in CML, there have been random reports of treatment failure or suboptimal response. Evidence suggests that individual variability in imatinib pharmacokinetics is crucial in optimizing the drug response. Hence, treatment of CML with imatinib should be guided through routine monitoring of its plasma levels.
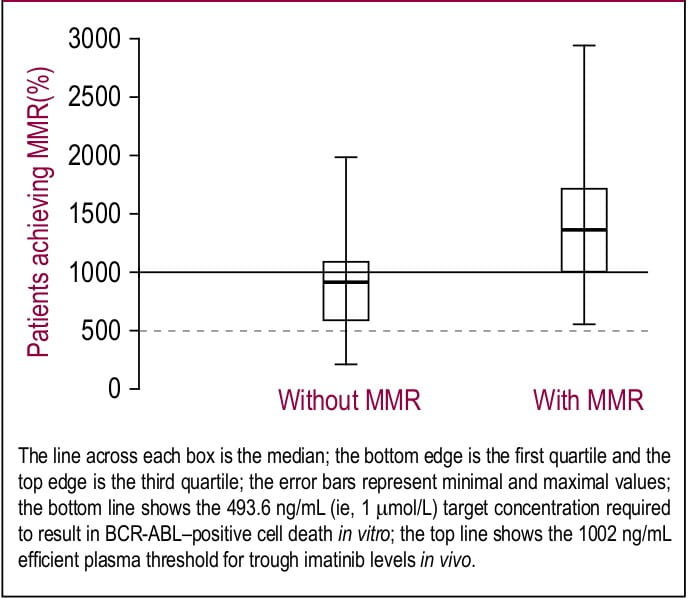
Picard et al, evaluated trough plasma concentration of imatinib in patients (n=68) with CML with or without response to imatinib (400 or 600 mg/day) for at least 12 months. The findings of the study were:
Therapeutic drug monitoring could be a valuable approach for identifying the reason of treatment failure or optimizing dose of imatinib.
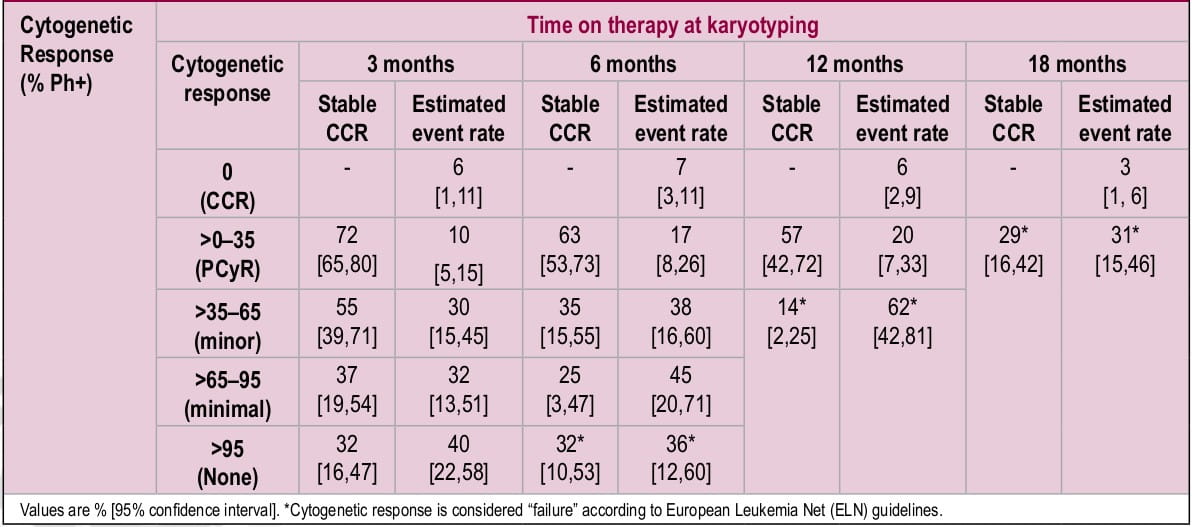
Figure 1. Trough plasma imatinib threshold for major molecular response (MMR)
Reference
1. Blood. 2007;109(8):3496-9
ISTAHIT is the first randomized phase III trial, wherein high-dose imatinib therapy improved MCR, CCR and MMR in patients with pre- treated chronic phase CML.
Imatinib 400 mg/day is used as standard treatment for patients with chronic phase CML. However, recent phase III clinical studies suggest that higher doses of imatinib (up to 800 mg/day) would improve response rates when carefully monitored for dose-related toxicities.1,2
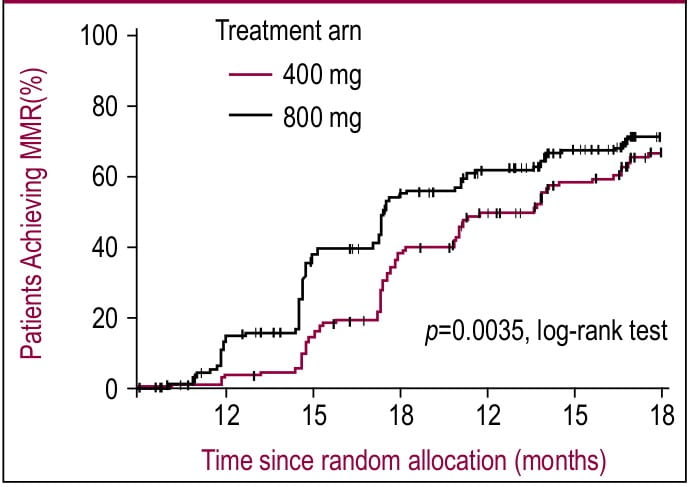
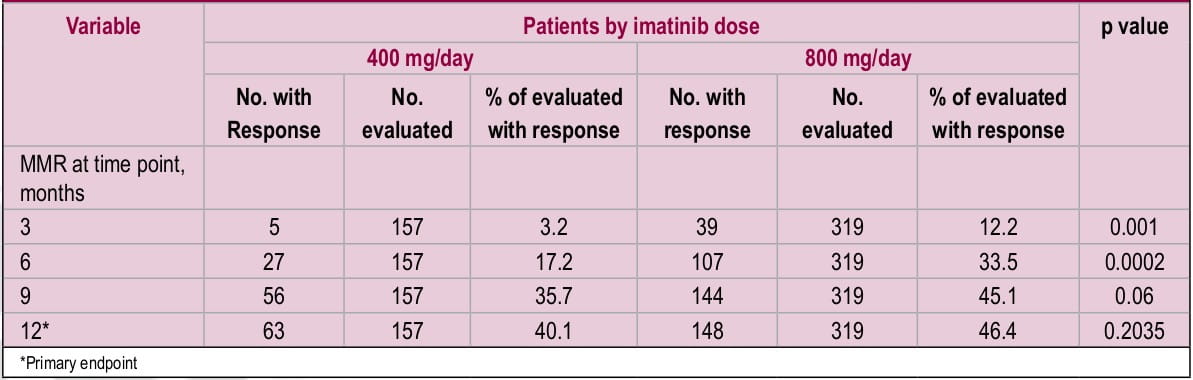
In ISTAHIT study, 227 patients with pre-treated Philadelphia chromosome-positive, BCR-ABL- positive CML were randomized to receive either standard-dose imatinib (400 mg/day) or a high- dose imatinib (800 mg/day for 6 months followed by standard dose maintenance therapy). Three and six month follow-up showed significantly high response rates in high-dose imatinib arm compared to standard dose [at 6 months; MCR: 54 vs. 34%, p=0.009; CCR: 44 vs. 20%, p<0.001].1 However, MCR was comparable between two arms at 12 months. Similarly, another study with a bigger sample size (n=476) showed that MMR at 12 months (primary endpoint) was not significantly different between two doses.2 However, among patients who had higher rates of MMR at 3 and 6 months, high- dose imatinib treatment resulted in faster achievement of MMR compared to standard dose imatinib (p=0.0035 by log-rank test; Figure 1 and Table 1).2 In both studies, WHO grade 3 and 4 hematologic toxicities were more common in the high-dose imatinib arm.
Current evidence suggests that high dose imatinib treatment could achieve faster MMR in responders.
However, long-term follow up studies are required to establish a clinical significance of this finding.
Figure 1. Time to first major molecular response (MMR) by treatment arm (intent-to-treat analysis).
References
1. Haematologica. 2010;95(6):908-13.
2. J Clin Oncol. 2010;28(3):424-30.
Researchers observed that imatinib therapy can cure CML in certain subset of patients without molecular relapse. However, discontinuation of imatinib therapy should be guided by individual response to therapy.
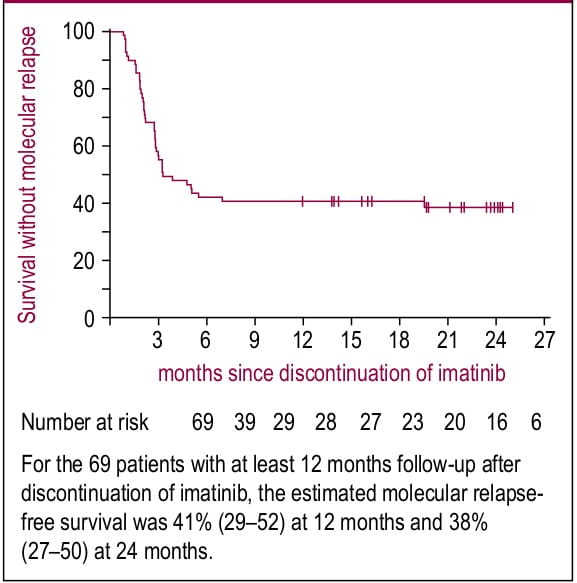
In a prospective, non-randomized STIM study, imatinib treatment was discontinued after >2 years treatment in CML patients (n=69) who were under CMR(>5-log reduction in BCR-ABL and ABL levels and undetectable transcripts on RT-PCR).1 After a median follow-up of 24 months (at least 12 months), 41% patients showed persistent CMR (95% CI: 29-52; Figure 1). Further, in patients who relapsed (61%) had responded to imatinib rechallenge in terms of decrease in their BCR-ABL levels (16 out of 42 patients) or achievement of CMR (26 out of 42 patients).1
In patients with a CMR of at least 2 years duration imatinib can be safely discontinued without the risk of molecular relapse.
Figure 1. Kaplan-Meier estimates of CMR after discontinuation of imatinib in patients with CML
Reference
1. Lancet Oncol. 2010;11(11):1029-35.
Ara-C: Cytarabine; ALL: Acute lymphoblastic leukemia; CCR: Complete cytogenetic response; CHR: Complete hematological response; CMDs: Chronic myeloid disorders; CML: Chronic myeloid leukemia; CR: complete remission; EFS: Event- free survival; HSCT: Hematopoietic stem cell transplantation; INF: Interferon; IRIS: International Randomized Study of Interferon and STI571; ISTAHIT: Imatinib Standard Dose versus High Dose Induction Trial; MCR: Major cytogenetic response; PDGF: Platelet-derived growth factor; PCyR: Partial cytogenetic response; RT-PCR: Real-time quantitative reverse transcriptase polymerase chain reaction; SHR: sustained hematological response; STIM: Stop Imatinib study