In pregnant women with confirmed HIV infection, the initiation of ART for maternal health is recommended for:
Preventing Mother-To-Child Transmission of HIV (PMTCT)
Preventing Mother-To-Child Transmission of HIV
(WHO 2010 Recommendations)
What's New?
2 Key Approaches
- For HIV-infected women in need of treatment for their own health
Lifelong antiretroviral therapy (ART) for HIV-infected women in need of treatment for their own health, which is also safe and effective in reducing mother-to-child transmission (MTCT) - For HIV-infected women not in need of treatment for their own health
Antiretroviral (ARV) prophylaxis to prevent MTCT during pregnancy, delivery and breastfeeding for HIV-infected women not in need of treatment
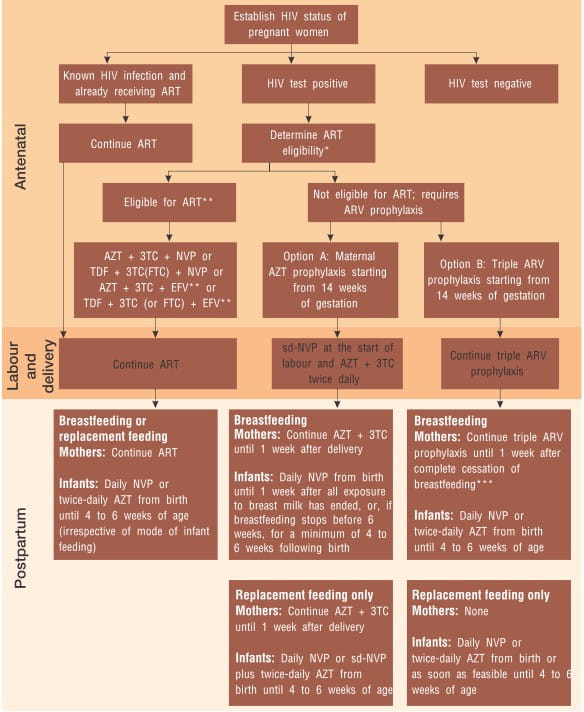
Pregnant Women Eligible for ART
When is ART Indicated
-
- all women with CD4 cell counts of <350 cells/mm,3irrespective of the WHO clinical staging
- all women in WHO clinical stage 3 or 4, irrespective of the CD4 cell count.
The above criteria for initiation are the same as for non-pregnant women.
When to Start ART in Pregnancy
HIV-infected pregnant women in need of ART for their own health should start ART as soon as feasible regardless of gestational age and continue throughout pregnancy, childbirth, breastfeeding (if breastfeeding), and thereafter.
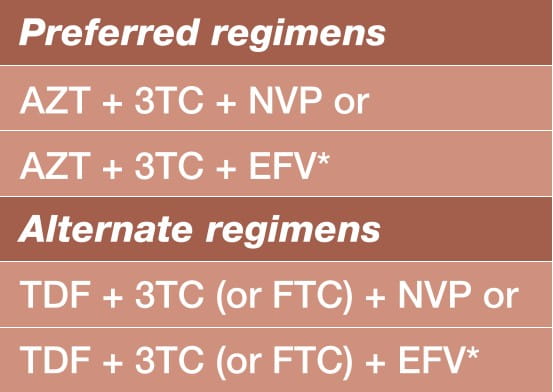
What ART Regimen to Initiate in the Mother
Choose one of the following regimens for maternal antepartum daily ART, starting as soon as possible irrespective of gestational age, and continued during pregnancy, delivery and thereafter.
What ARV Prophylaxis to Give Infants of HIV-Infected Women Receiving ART
All infants (regardless of whether breastfeeding or receiving only replacement feeding) born to HIV-infected women receiving ART for their own health should be given daily NVP or twice-daily AZT from birth or as soon as feasible thereafter until 4 to 6 weeks of age.
ARV Prophylaxis for Pregnant Women Who Do Not Need Treatment for Their Own Health
HIV-infected pregnant women who are not in need of ART for their own health require effective ARV prophylaxis to prevent HIV infection in their infants. ARV prophylaxis should be started from as early as 14 weeks of gestation (second trimester) or as soon as feasible during pregnancy, labour and delivery or thereafter.
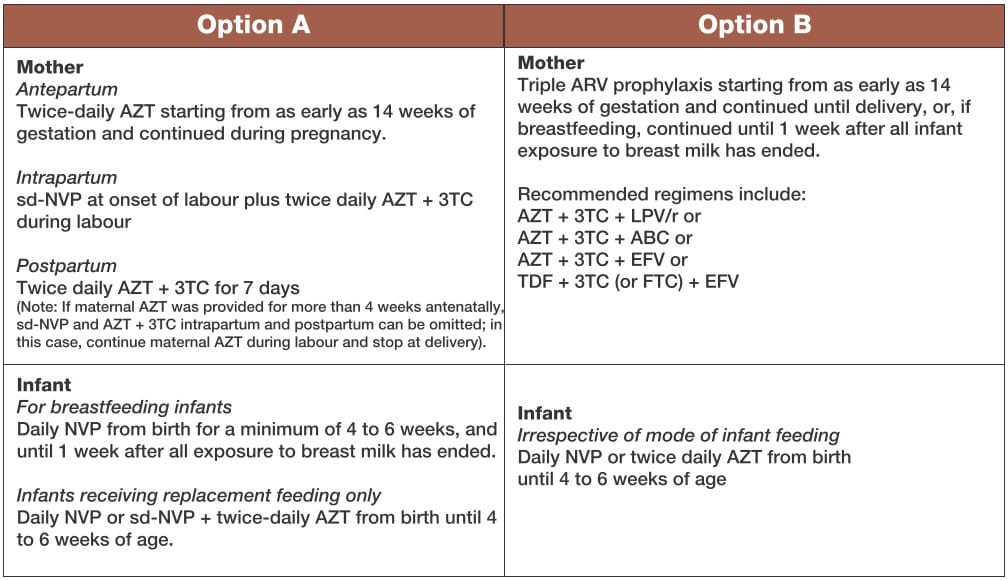
What ARV Prophylaxis Regimen to Give Women and Their Infants
The two options A and B are outlined below:
Both recommended options provide a significant reduction in the risk of MTCT. There are advantages and disadvantages of both options, in terms of feasibility, acceptability and safety for mothers and infants, and cost.
To What Extent Can these Regimens Reduce Risk of Vertical Transmission ?
Effective Implementation of These Recommendations will Ensure Increased Maternal and Child Survival
- In breastfeeding populations, the risk of MTCT can be reduced to less than 5% (or even lower) from a background risk of 35%.
- In non-breastfeeding populations, this could reduce to less than 2% from a background risk of 25%.