Opsutan (Macitentan) Monograph
Pulmonary Arterial Hypertension: Overview
Pulmonary arterial hypertension (PAH) is a chronic and progressive disease, characterised by the presence of precapillary pulmonary hypertension.1 PAH presents with elevated pressure in the pulmonary artery in the setting of normal or reduced cardiac output and normal pulmonary capillary wedge pressure.2The World Health Organisation (WHO) defines PAH as mean pulmonary arterial pressure (mPAP) >25 mmHg at rest, pulmonary vascular resistance (PVR) >3 Wood units, and pulmonary capillary wedge pressure (PCWP) ≤15 mmHg. The disease is classified based on the aetiology of PAH as well as severity of symptoms (WHO functional class [FC] I—IV)3(see Tables 1 and 2).
|
1. PAH 1.1 Idiopathic PAH 1.2 Heritable PAH 1.2.1 BMPR2 1.2.2 ALK-1, ENG, SMAD9, CAV1, KCNK3 1.2.3 Unknown 1.3 Drug- and toxin-induced 1.4 Associated with: 1.4.1 Connective tissue disease 1.4.2 HIV infection 1.4.3 Portal hypertension 1.4.4 Congenital heart diseases 1.4.5 Schistosomiasis 1’ Pulmonary veno-occlusive disease (PVOD) and/or pulmonary capillary haemangiomatosis 1” Persistent pulmonary hypertension of the newborn BMPR, bone morphogenic protein receptor type II; CAV1, caveolin-1; ENG, endoglin; PAH, pulmonary arterial hypertension |
|
Table 2: WHO classification of functional status of patients with pulmonary
hypertension (modified from the New York Heart Association functional classification)
Class and Description I Patients with pulmonary hypertension but without resulting limitation of physical activity. Ordinary physical activity does not cause undue dyspnoea or fatigue, chest pain, or near syncope. II Patients with pulmonary hypertension resulting in slight limitation of physical activity. They are comfortable at rest. Ordinary physical activity causes undue dyspnoea or fatigue, chest pain, or near syncope. III Patients with pulmonary hypertension resulting in marked limitation of physical activity. They are comfortable at rest. Less than ordinary physical activity causes undue dyspnoea or fatigue, chest pain, or near syncope. IV Patients with pulmonary hypertension with inability to carry out any physical activity without symptoms. These patients manifest signs of right heart failure. Dyspnoea and/or fatigue may even be present at rest. Discomfort is increased by any physical activity. |
Elevated PVR and the resulting increased right ventricular (RV) afterload contribute to the maladaptive processes of RV hypertrophy and dilatation, which eventually result in RV failure due to the inability of RV contractility to adapt to the increasing afterload3 and premature death. Other hallmarks of RV impairment during disease progression include reduced cardiac index (CI), increased right atrial pressure (RAP), and elevated levels of N-terminal pro-brain natriuretic peptide (NT-pro-BNP).4
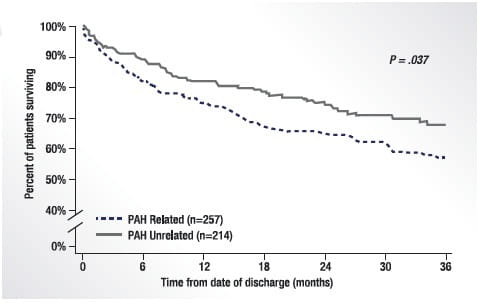
The progressive nature of PAH means that morbidity events associated with the disease require hospitalisation. Hospitalisation has a negative impact on patients’ quality of life and an adverse effect on the lives of their carers, and represents a substantial healthcare burden. Data from the REVEAL (Registry to Evaluate Early and Long-term PAH Disease Management) Registry showed that patients who were hospitalised for PAH were more likely to be rehospitalised and had worse survival at 3 years than those who were not hospitalised (Fig. 1). 5
A Look at the Numbers
Registry data from across the world suggest that the epidemiology of PAH has changed dramatically over the past three decades. In the landmark National Institutes of Health (NIH) Registry conducted in the 1980s, PAH patients were predominantly young (mean age of 36 years at presentation) and female (1.7:1) and had idiopathic or familial PAH, with 1-, 3- and 5-year survival of 67, 45 and 37%, respectively. More recent PAH registries including the REVEAL Registry, the Pulmonary Hypertension connections (PHC-single-centre US-based registry), and the French National Registry indicate older (mean age at presentation ranging from 48 to 53 years) patients with improved survival rates.6
Aetio-Pathogenesis of PAH
PAH results from functional and structural changes in the pulmonary vasculature, leading to increased PVR, RV failure and, subsequently, death2.
Imbalances in three main biological pathways — the prostacyclin, nitric oxide and endothelin (ET)-1 pathways — have been implicated in the pathogenesis of PAH2(Fig. 2).
Endothelial dysfunction is typical in PAH and involves an increased production of vasoconstrictive compounds such as endothelin and thromboxane and decreased production of vasodilatory compounds such as prostacyclin and nitric oxide. Each substance may mediate multiple effects (Fig 2).3
Endothelin Receptor Antagonist Pathway
ET-1, a potent vasoconstrictor, has been implicated in playing a significant role in the progression of pulmonary vasculopathy in PAH patients.7 Increased levels of ET-1 have been seen in the plasma and pulmonary vascular endothelium of patients with PAH, indicating that ET-1 is likely to contribute to the vasodilator/vasoconstrictor imbalance associated with PAH.2ET-1 is released mainly from the basal surface of the endothelium and acts largely on the underlying smooth muscle cells, resulting in vasoconstriction and proliferation. ET-1 mediates its effects via two receptor subtypes, the ETA receptors (expressed on smooth muscle cells, fibroblasts and cardiac myocytes) and the ETB receptors (expressed predominantly on endothelial cells and, to a lesser extent, on vascular smooth muscle cells, fibroblasts and macrophages).2
Upregulation of ET-1 and its receptors, ETA and ETB, leads to increased PVR and hypertrophy. Plasma levels of ET-1 have been found to correlate with severity of PAH and prognosis.3
Endothelin receptor antagonists (ERAs) include bosentan, ambrisentan and macitentan. While ambrisentan selectively binds to ETA, bosentan and macitentan are dual ERAs that bind to both ETA and ETB.8
ERAs, along with prostanoids (targeting the prostacyclin pathway) and phosphodiesterase-5 inhibitors ([PDE5i] targeting the nitric oxide pathway), are the mainstay of the current treatment strategies for PAH.2
Macitentan
Macitentan is a dual ERA approved for the long-term treatment of PAH.
Chemistry
IUPAC name
5-(4-bromophenyl)-6-[2-(5-bromopyrimidin-2-yl)oxyethoxy]-N-(propylsulphamoyl)pyrimidin-4-amine8
Pharmacodynamics
Macitentan is a nonselective or dual ERA that inhibits binding of ET-1 to the ETA and ETB receptors. It is a lipophilic sulphamide compound designed to decrease vascular resistance by exhibiting enhanced tissue penetration and prolonged receptor binding to ETA/ETB in pulmonary arterial smooth muscle cells. Its ETA/ETB inhibitory potency ratio has been found to be 50:1 in functional assays in isolated organs. The pharmacological effect of macitentan is achieved by the combined actions of the parent compound and its active metabolite, ACT-132577.3
Data from in vitro and in vivo studies have demonstrated macitentan’s higher affinity for lipid and tissue compared with its structural precursor bosentan and the ETA selective antagonist, ambrisentan. Macitentan partitions 40- and 2,000-fold better into lipids than bosentan and ambrisentan, respectively, as seen in in vitro studies.2
ACT-132577 is also pharmacologically active at these receptors (ETA/ETB inhibitory potency ratio was 16:1) and is ~20% as potent as the parent compound.2
Macitentan displayed slower receptor dissociation kinetics than ambrisentan and bosentan (receptor occupancy half-life of ~~17 min versus ~40 and~70 s) and, unlike ambrisentan and bosentan, blocked ET-1 receptor activation across a wide range of ET-1 concentrations, suggesting a more complete block of ET-1 binding under conditions of locally fluctuating ET-1 levels.2
Pharmacokinetics
In healthy individuals, the pharmacokinetics of macitentan was shown to be dose-proportional over a range of 1 to 30 mg, taken once daily.
Men aged 20 to 50 years with a body mass index of 18 to 28 kg/m2 were randomised to receive either macitentan (1, 3, 10 or 30 mg) or placebo in a double-blind fashion. Macitentan was administered once daily for 10 days; participants were assessed after each dose.2
Plasma concentrations of macitentan and ACT-132577 were measured as well as plasma concentrations of ET-1 and urinary levels of 6β-hydroxycortisol and cortisol.2
- The plasma concentration of macitentan increased proportionally across doses during the study period.
- The median time to maximum plasma concentration of macitentan was approximately 8 hours.
- Accumulation of the parent compound was estimated at a ratio of 1.5 for the different doses; in contrast, accumulation of ACT-132577 was higher (ratios between 7.1 and 9.9).
Patients with PAH had exposures to the parent compound and active metabolite similar to those seen in healthy participants.2
The absolute bioavailability is unknown, but exposure to both the drug and its active metabolite are not affected by food.
The apparent volume of distribution is 50 and 40 L for macitentan and its active metabolite, respectively.2
Both are highly protein-bound (>99%) in plasma, mostly to albumin, with some binding to α-1-acid glycoprotein.2
The disposition and metabolism of macitentan were evaluated in another study in healthy individuals in which men aged 45 to 65 years with body mass index in the range of 18 to 28 kg/m2 were given a single dose of 14C-labelled macitentan 10 mg and monitored for 14 days.2
- The parent compound and active metabolite, ACT-132577, as well as an inactive metabolite, ACT-373898, were identified in the plasma, urine and faeces.
- Metabolism occurred primarily through the cytochrome P450 (CYP) 3A4 isoenzyme with CYP2C19 being a minor pathway.
- Urine was determined to be the major elimination route for both the active and inactive metabolites.
- The mean elimination half-life of macitentan was 15.0 hours, whereas that of its active metabolite was 44.0 hours.
Pharmacokinetics in patients with hepatic impairment
In patients with mild, moderate or severe hepatic impairment (Child-Pugh Class A, B, or C), the area under the plasma concentration time curve from zero to infinity (AUC∞) for macitentan was reduced compared with that of healthy individuals, following a single oral dose of 10 mg.2
Pharmacokinetics in patients with renal impairment
In the same comparison, AUC∞ for the active metabolite was also reduced. In patients with severe renal impairment (creatinine clearance 15—29 mL/min), the mean AUC∞ for macitentan and its active metabolite were both higher compared with those of healthy individuals.2
Clinical Efficacy of Macitentan
Introduction to the Landmark SERAPHIN Trial
(Study with an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcome)
|
SERAPHIN is the first ERA trial to use an event-driven strategy with a composite primary endpoint of morbidity or mortality. Previous trials have focused on short-term outcomes, such as improved 6-minute walk distance (6MWD) and WHO-FC. |
SERAPHIN was a randomised, double-blind, multinational, Phase 3 trial that examined the efficacy of macitentan in patients aged >12 years with PAH (confirmed by right heart catheterisation), a 6MWD of >50 m, and a WHO-FC of II, III or IV.9
A total of 742 patients were randomly assigned to placebo, macitentan 3 mg and macitentan 10 mg once daily in a 1:1:1 ratio.
Primary Endpoints
The composite primary endpoint was the time from the initiation of treatment to the first event related to PAH, i.e.
- worsening of PAH;
- initiation of treatment with intravenous (IV) or subcutaneous (SC) prostanoids; and/or
- lung transplantation, or atrial septostomy or death from any cause, up to the end of treatment.
Worsening of PAH was defined by the occurrence of all three of the following:
- A decrease in the 6MWD of at least 15% from baseline, confirmed by a second 6MWD test performed on a different day within 2 weeks.
- Worsening of symptoms of PAH (including at least one of the following: a change from baseline to a higher WHO-FC (or no change in patients who were in WHO-FC IV at baseline) and the appearance or worsening of signs of right heart failure that did not respond to oral diuretic therapy.
- Need for additional treatment for PAH.
Secondary Endpoints
Prespecified secondary endpoints included the change from baseline to month 6 in the 6MWD, the percentage of patients with an improvement in WHO-FC at month 6, death due to PAH or hospitalisation for PAH up to the end of treatment, and death from any cause up to the end of treatment and up to the end of the study.
Safety Endpoints
Safety endpoints included adverse events and laboratory abnormalities.
Results
- The baseline patient profile
PAH was idiopathic in 55.0% of patients, heritable in 1.8%, and related to connective tissue disease, repaired congenital systemic-to-pulmonary shunts, HIV infection, or drug use/toxin exposure in 30.5, 8.4, 1.4 and 3.0% of patients, respectively. Patients were treatment-naïve (36.3% of patients) or had been receiving stable background treatment for >3 months (63.7%), including with oral PDE5i (61.4%) or oral or inhaled prostanoids (5.4%).
At baseline, mean patient age was 45.6 years, the mean 6MWD was 360 m, and WHO-FC I, II, III or IV was seen in 0.1, 52.4, 45.6 and 1.9% of patients, respectively.
- Primary endpoint
A total of 287 patients had a primary endpoint event over a median treatment period of 115 weeks: 116 patients (46.4%) in the placebo group, 95 patients (38.0%) in the group that received 3 mg of macitentan, and 76 patients (31.4%) in the group that received 10 mg of macitentan.
Worsening of PAH was the most frequent primary endpoint event.
The hazard ratio [HR] for the primary endpoint with the 3-mg dose of macitentan versus placebo was 0.70 (97.5% confidence interval [CI], 0.52 to 0.96; P=0.01 by the log-rank test), and the HR with the 10-mg dose of macitentan versus placebo was 0.55 (97.5% CI, 0.39 to 0.76; P<0.001 by the log-rank test) (Fig. 4).
Kaplan–Meier estimates for the first event related to PAH (worsening of PAH, initiation of treatment with IV or SC prostanoids, lung transplantation, or atrial septostomy) or death from any cause show a significant treatment effect in favour of macitentan at a once-daily dose of 3 mg versus placebo (HR, 0.70; 97.5% CI, 0.52 to 0.96; P=0.01 by the log-rank test) and macitentan at a once-daily dose of 10 mg versus placebo (HR, 0.55; 97.5% CI, 0.39 to 0.76; P<0.001 by the log-rank test).
The composite endpoint of death due to PAH or hospitalisation for PAH occurred in 84 patients (33.6%) in the placebo group, 65 patients (26.0%) in the group that received 3 mg of macitentan, and 50 patients (20.7%) in the group that received 10 mg of macitentan), with hospitalisation accounting for most of these events. The hazard ratio with the 3 mg dose of macitentan versus placebo was 0.67 (97.5% CI, 0.46 to 0.97; P=0.01 by the log-rank test), and the HR with the 10 mg dose of macitentan versus placebo was 0.50 (97.5% CI, 0.34 to 0.75; P<0.001 by the log-rank test) (Fig. 5).
- Secondary endpoint
Exercise capacity and FC
At month 6, the 6MWD had decreased by a mean of 9.4 m in the placebo group. In contrast, the 6MWD had increased by a mean of 7.4 m in the group that received 3 mg of macitentan (treatment effect with 3 mg dose versus placebo, 16.8 m; 97.5% CI, −2.7 to 36.4; P=0.01) and by a mean of 12.5 m in the group that received 10 mg of macitentan (treatment effect with 10 mg dose versus placebo, 22.0 m; 97.5% CI, 3.2 to 40.8; P=0.008).
The WHO-FC improved from baseline to month 6 in 13% of the patients in the placebo group, as compared with 20% of those in the group that received 3 mg of macitentan (P=0.04) and 22% of those in the group that received 10 mg of macitentan (P=0.006).
|
Key highlights of the study Macitentan significantly reduced morbidity and mortality among patients with PAH. Benefits were shown both for patients who had not received treatment previously and for those receiving therapy for PAH at study entry. |
Effect of Macitentan on Haemodynamic Parameters: A SERAPHIN Substudy
The effect of macitentan on haemodynamic parameters and NT-pro-BNP levels was evaluated in PAH patients in the SERAPHIN study. The association between these parameters and disease progression, assessed by the primary endpoint (time to first morbidity/mortality event), was explored.5
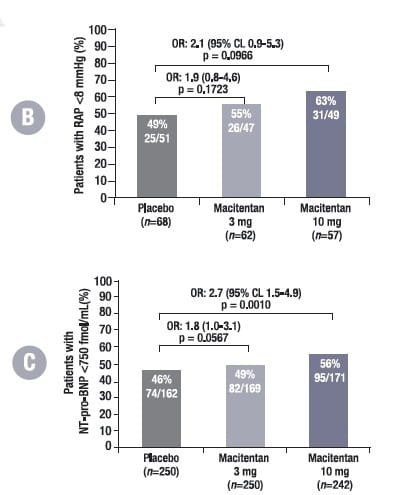
Of the 187 haemodynamic sub-study patients, 68 were randomised to placebo, 62 to macitentan 3 mg, and 57 to macitentan 10 mg. The majority were in WHO-FC II and III, and half of the patients were on background PAH-specific therapy, mainly sildenafil.
In clinical practice, the goal of the PAH physician is to maintain or bring his/her patient to a low-risk profile, part of which includes achieving and/or maintaining good RV function.
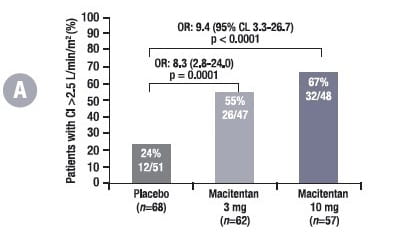
In this sub-study, at month 6, patients treated with macitentan were more likely to have a CI >2.5 L/min/m2, RAP <8 mmHg and NT-pro-BNP <750 fmol/ml compared with placebo-treated patients (Fig. 6), which further lowers the risk of disease progression compared with patients who did not achieve this.
Adjusted for values at baseline. For CI and RAP, n denotes the number of patients who participated in the haemodynamic sub-study; for NT-pro-BNP, n denotes all randomised patients in the SERAPHIN study. The percentages are proportions of patients with values at month 6 who reached the respective thresholds.
OR, odds ratio; CL, confidence limit; CI, cardiac index; RAP, right atrial pressure; NT-pro-BNP, N-terminal pro-brain natriuretic peptide
After 6 months, patients with a CI > 2.5 L/min/m2 vs. a CI < 2.5 L/min/m2 had a 51% reduction in the risk of morbidity/mortality events. Patients with RAP < 8mmHg vs. RAP > 8mmHg at month 6 had a 28% reduction in the risk of morbidity/mortality and patients with NT-proBNP < 750 fmol/ml vs. > 750 fmol/ml at month 6 had a 78% reduction in the risk of morbidity/mortality.
Macitentan improved haemodynamic parameters irrespective of WHO-FC and background PAH-specific therapy.
|
Key highlights of the study
|
Effect of Macitentan on Hospitalisations: Post-hoc Analysis from SERAPHIN
The effects of macitentan on the time to first all-cause hospitalisation and time to first PAH-related hospitalisation up to the end of treatment were assessed by post-hoc analyses of all patients.4
Results
All-cause hospitalisation
There were 117 (46.8%), 104 (41.6%) and 90 (37.2%) patients in the placebo, macitentan 3 mg and 10 mg arms, respectively, who were hospitalised for any cause at least once during double-blind treatment, and they experienced a total of 171, 159 and 135 all-cause hospitalisations, respectively.
- Risk of all-cause hospitalisation: Compared with that of placebo, the risk of all-cause hospitalisation with 3 mg of macitentan was reduced by 18.9% (HR: 0.811; 95% CI: 0.623 to 1.057; p=0.1208) and with 10 mg of macitentan by 32.3% (HR: 0.677; 95% CI: 0.514 to 0.891; p=0.0051) (Fig. 7).
- Rates of all-cause hospitalisation: Rates of all-cause hospitalisation per 100 patient-years were 41.5, 33.0 and 27.7 in the placebo, macitentan 3 mg, and 10 mg arms, respectively (see Table 3).
- Mean number of hospital days for all-cause hospitalisations: The mean number of hospital days for all-cause hospitalisations were 4.1, 2.9 and 2.8 days in the placebo, macitentan 3 mg, and 10 mg arms, respectively (see Table 3).
|
|
Placebo |
Macitentan 3 mg |
Macitentan 10mg |
|
Rates of all-cause hospitalisation per 100 patient-years |
41.5 |
33.0 |
27.7 |
|
|
Rates of hospitalisation reduced by 20.5% compared with placebo (p=0.0378) |
|
|
|
|
|
||
|
|
|
|
Rates of hospitalisation reduced by 33.1% compared with placebo (p=0.0005). |
|
Mean number of hospital days for all-cause hospitalisations |
4.1 days |
2.9 days |
2.8 days |
|
|
The mean number of hospital days was reduced by 30.6% with 3 mg of macitentan (p=0.0278) compared with placebo
|
|
|
|
|
|
|
The mean number of hospital days was reduced by 31% with 10 mg of macitentan (p=0.0336) compared with placebo
|
PAH-related hospitalisation
There were 80 (32.0%), 53 (21.2%) and 46 (19.0%) patients in the placebo, macitentan 3 mg, and 10 mg arms, respectively, who were hospitalised at least once for PAH during the double-blind treatment period, and they experienced a total of 91, 59 and 54 PAH related hospitalisations, respectively.
Risk of PAH-related hospitalisation: Compared with placebo, the risk of PAH-related hospitalisation was reduced by 42.7% in patients treated with 3 mg of macitentan (HR: 0.573; 95% CI: 0.405 to 0.811; p =0.0015) and by 51.6% in patients treated with 10 mg of macitentan (HR: 0.484; 95% CI: 0.337 to 0.697; p<0.0001) (Fig 8).
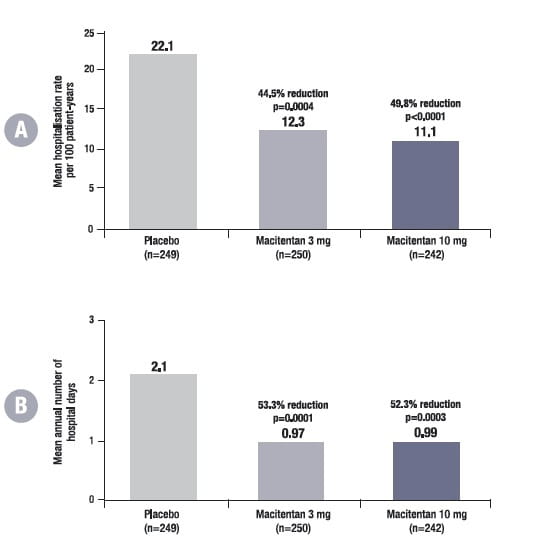
Rate of PAH-related hospitalisation: Compared with placebo, the rate of PAH-related hospitalisation was reduced by 44.5% in the macitentan 3 mg arm (p = 0.0004) and by 49.8% in the 10-mg arm (p<0.0001) (Fig. 9A).
Mean number of hospital days for PAH-related hospitalisations: The mean number of annual hospital days for PAH-related hospitalisations was reduced by 53.3% in the 3 mg arm (p=0.0001) and by 52.3% in the 10 mg arm (p=0.0003) (Fig. 9B).
|
Key highlights of the study
|
Macitentan Improves Health-related Quality of Life for Patients with PAH: Evaluation of Patients from SERAPHIN
Since PAH impacts the daily activities of patients and reduces their health-related quality of life (HRQoL) not only due to the symptoms but also due to the social, emotional and psychological aspects, the impairment experienced in PAH is comparable with other serious conditions, including COPD and renal failure; hence, SERAPHIN included an evaluation of HRQoL among patients with PAH.10
Results
- SERAPHIN, using the SF-36, is the first study to demonstrate a benefit of PAH-targeted therapy in seven of the eight domains (Fig. 10).
- Macitentan also improved both the physical and mental component summary scores.
- The improvement in HRQoL in patients receiving macitentan is paralleled by improved functional parameters such as 6MWD and WHO-FC.
- In addition, macitentan’s convenient oral administration and its well-tolerated adverse event profile may contribute to the HRQoL benefits, compared with therapies that require parenteral administration or close monitoring for side-effects.
Macitentan in PAH: A Focus on Combination Therapy in the SERAPHIN Trial
Use of combination therapy for PAH is on the rise, but —until recently — evidence of its benefits from long-term clinical studies was lacking.
The SERAPHIN study enrolled large number of patients who were taking background PAH therapy (primarily PDE5i), allowing examination of the efficacy and safety of macitentan in combination therapy.11
Baseline characteristics of patients receiving background therapy
In total, 154 patients randomised to macitentan 10 mg and 154 randomised to placebo were receiving background PAH therapy. Background therapy consisted primarily of a PDE5i (97.4%) in both the macitentan and placebo groups; overall, 5.4% of patients were receiving in addition an inhaled or oral prostacyclin.
Effect of combination therapy with macitentan on the risk of morbidity/mortality
The risk of morbidity/mortality was reduced by 38% in patients who received macitentan and background therapy compared with those receiving background therapy alone (placebo) (HR, 0.62; 95% CL, 0.43 to 0.89; p=0.009) (Fig. 11).
These data are the first randomised, controlled, trial-based evidence demonstrating that combination therapy improves long-term outcomes in PAH.
Safety and tolerability of combination therapy with macitentan
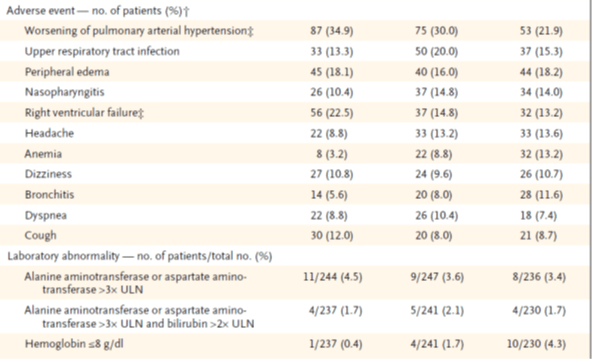
Adverse events experienced by patients receiving macitentan plus background therapy were similar to those recorded for patients receiving background therapy alone (table 4). The number of patients in the placebo, 3-mg macitentan, and 10-mg macitentan groups who discontinued the study drug owing to adverse events was 31 (12.4%), 34 (13.6%), and 26 (10.7%), respectively.
|
Placebo Macitentan 3 mg Macitentan 10 mg |
† Adverse events listed are those that occurred in more than 10% of the patients in any study group during the treatment period and up to 28 days after discontinuation of the study drug.
‡ The majority of these events were also reported as primary endpoint events.
Key highlights of the study
- Combination therapy with macitentan is safe and well-tolerated in the management of PAH. It significantly reduces mortality and morbidity events like worsening due to PAH and risk and rates of hospitalisation.
Macitentan for the Treatment of Inoperable Chronic Thromboembolic Pulmonary Hypertension (MERIT-1)
The microvasculopathy of chronic thromboembolic pulmonary hypertension (CTEPH) and PAH are similar. MERIT 1 was a Phase 2, double-blind, randomised, placebo-controlled that assessed macitentan in 80 patients with CTEPH adjudicated as inoperable.12 Patients identified as WHO-FC II—IV with a PVR of at least 400 dyn∙s/cm⁵ and a 6MWD of 150—450 m were randomly assigned (1:1), via an interactive voice/web response system, to receive oral macitentan (10 mg once a day) or placebo. Treatment with PDE5i and oral or inhaled prostanoids were permitted for WHO-FC III/IV patients.
The primary endpoint was resting PVR at week 16, expressed as percentage of PVR measured at baseline.
Results
At week 16
- The geometric mean PVR at rest decreased to 73·0% (95% CI, 63·6 to 83·8) of the baseline value in the macitentan group, corresponding to a mean decrease from baseline of 206 dyn∙s/cm⁵, and decreased to 87·2% (95% CI, 78·5 to 96·7) of the baseline value in the placebo group, corresponding to a mean decrease from baseline of 86 dyn·s/cm⁵ (ratio of geometric means 0·84, 95% CI, 0·70 to 0·99, p=0·041) (Fig. 12).
- The exploratory endpoint of cardiac output increased from baseline by a mean of 0·76 L/min in the macitentan group while decreased from baseline by a mean of 0·02 L/min in the placebo group (p=0·0005).
At week 24
- The 6MWD increased from baseline by a mean of 35·0 m (SD, 52·52) in the macitentan group versus 1·0 m (83·24) in the placebo group (p=0·033) (Fig. 13).
- There was no significant difference between the macitentan group and the placebo group in the Borg dyspnoea score (p=0·35) and none of the 40 patients in the macitentan group had a worsening in WHO-FC, whereas 3 (7·5%) of 40 patients in the placebo group had a worsening in WHO-FC (OR, 0·21; 95% CI, <0·001 to 1·46; p=0·096).
- The geometric mean NT-pro-BNP concentration decreased to 72·6% of the baseline value (mean decrease from baseline of 651 pg/mL) in the macitentan group compared with 90·9% of the baseline value (mean decrease from baseline of 360 pg/mL) in the placebo group (ratio of geometric means, 0·79; 95% CI, 0·63 to 0·99; p=0·040).
- Up to 95·0% (95% CI, 81·5 to 98·7) of patients receiving macitentan and 87·5% (95% CI, 72·5 to 94·6) of patients receiving placebo were free from pulmonary hypertension-related disease progression (p=0·085).
Macitentan, thus, significantly improved cardiopulmonary haemodynamics and clinical variables in patients with inoperable CTEPH.
Switch from Bosentan to Macitentan in Patients with PAH-CHD
In a prospective observational study13, in 43 adult PAH-CHD patients, previously treated with bosentan for a median treatment duration of 7.2 years (interquartile range (IQR) 5.0 to 8.1), a switch to macitentan was performed with a 24-hour wash-out period after bosentan cessation.
Of the 43 PAH-CHD patients on bosentan, 40 patients (45 ± 13 years, 40% male, 40% Down syndrome, 75% Eisenmenger’s) were willing to switch to macitentan. The following parameters were assessed: hospitalisation for heart failure, syncope, WHO-FC III or IV, 6MWD, oxygen saturation (SaO2), ferritin, NT-pro-BNP, and tricuspid annular plane systolic excursion (TAPSE).
Clinical status of patients prior to the switch
Before the therapy switch, the clinical status of the patients was as follows:
- 52% of patients were in WHO-FC II and 48% in class III
- Mean 6MWD was 394±125 m
- Most patients had a normal renal function (median creatinine, 80 μmol/l, IQR 81 to 93, upper limit of normal (ULN) 110), and elevated NT-pro-BNP (median 723 ng/l, IQR 311 to 1328, ULN 130).
Improvement seen in all parameters of assessment at 6 months post-switch
The clinical outcome in 40 PAH-CHD patients who switched from bosentan to macitentan is presented in Table 5.
The switch from bosentan to macitentan was associated with improvement of WHO-FC, NT-pro-BNP and TAPSE, even after several years of bosentan. Macitentan therapy was well-tolerated.
|
n=40 |
Bosentan |
Macitentan |
P value |
|
Determinants of prognosis Hospitalisation heart failurea, % |
7.5 |
2.5 |
0.50 |
|
Syncope, % |
2.5 |
2.5 |
1.00 |
|
WHO-FC III or IV, % |
48 |
23 |
0.004 |
|
6-MWD, m ± SD |
394 ± 125 |
397 ± 123 |
0.79 |
|
SaO2, %, IQR |
87 81–93 |
85 81–94 |
0.42 |
|
Ferritin, ng/l, IQR |
45 22–89 |
63 28–110 |
0.41 |
|
NT-pro-BNP, ng/l, IQR |
723, 311–1328 |
488, 215–1291 |
0.02 |
|
TAPSE, mm ± SD |
19 ± 4 |
21 ± 5 |
0.002 |
PAH-CHD, pulmonary arterial hypertension due to congenital heart disease; SD, standard deviation; IQR, interquartile range; WHO, World Health Organization; 6MWD, six-minute walk distance; SaO2, arterial oxygen saturation; NT-pro-BNP, N-terminal pro-brain natriuretic peptide; TAPSE, tricuspid annular plane systolic excursion. Statistics used include McNemar's test, 2-tailed paired t-test or Wilcoxon signed-rank test, as appropriate. a Hospitalization for heart failure and syncope events were recorded during 6 months of bosentan and macitentan therapy.
This study is the first to describe the effect of a switch from bosentan to macitentan on clinical status in patients with PAH-CHD. All PAH-CHD subclasses were investigated, including patients with Eisenmenger’s syndrome.
The beneficial changes, apparent at 6 months’ follow-up, are explained by the sustained receptor binding and enhanced tissue penetration of macitentan compared to bosentan.
Safety and Tolerability of Macitentan
Macitentan is generally well-tolerated in patients with PAH and had a tolerability profile generally similar to that of placebo; the most common adverse events that occurred more frequently with macitentan than placebo were upper respiratory tract infection, headache and anaemia.
Hepatotoxicity and liver failure have been associated with other ERAs.
- Liver enzyme testing prior to initiation of treatment and continued monitoring as clinically warranted during treatment is recommended.
- Patients should be counselled to report symptoms associated with hepatotoxicity, and macitentan should be discontinued if symptoms are associated with elevated aminotransferases with or without elevated bilirubin above twice the ULN.
The incidences of elevations in liver enzyme levels of [3 times the ULN (class effects)] were similar between macitentan and placebo recipients in SERAPHIN study.
Haemoglobin and haematocrit reductions have occurred with the use of other ERAs.
- Baseline haemoglobin measurements should be performed, and haemoglobin should be monitored during treatment if clinically warranted.
- Macitentan should not be initiated in patients with severe anaemia.
However, compared to other ERAs, macitentan has lower risk for increase in liver enzymes, anaemia and peripheral oedema (Table 6).15
|
Adverse Effects
|
Bosentan
|
Ambrisentan
|
Macitentan
|
|
Aminotransferase elevations |
>10% of cases
|
<3% of cases |
<3% of cases |
|
Peripheral oedema |
3%–10% of cases |
>10% of cases
|
<3% of cases |
|
Anaemia |
3%–10% of cases |
3%–10% of cases
|
3%–10% of cases
|
Impaired spermatogenesis has been associated with other ERAs.
- Male patients should be counselled on the potential effects on fertility prior to initiation of treatment.
Macitentan carries a boxed warning for embryo-foetal toxicity and is contraindicated in pregnant women because of teratogenicity demonstrated in animal studies. Women of childbearing potential must adhere to required pre-treatment pregnancy testing and counselling, acceptable methods of contraception, and monthly pregnancy testing during and 1 month after discontinuing treatment with macitentan.
Indications, Dosage and Administration
OPSUTAN Tablets are indicated for the treatment of PAH (WHO Group I) to delay disease progression. Disease progression included death, initiation of IV or SC prostanoids, or clinical worsening of PAH (decreased 6MWD, worsened PAH symptoms, and need for additional PAH treatment).
Macitentan also reduces hospitalisation for PAH.
The recommended dosage of OPSUTAN Tablets is 10 mg once daily for oral administration. Doses higher than 10 mg once daily have not been studied in patients with PAH and are not recommended.
OPSUTAN Tablets should be taken every day at about the same time. If the patient misses a dose of OPSUTAN Tablets, the patient should be told to take it as soon as possible and then take the next dose at the regularly scheduled time. The patient should be told not to take two doses at the same time if a dose has been missed.
Pregnancy testing in females of reproductive potential
Initiate treatment with OPSUTAN Tablets in females of reproductive potential only after a negative pregnancy test. Obtain a monthly pregnancy test during treatment.
Warnings, Precautions and Contraindications
- ERAs cause hepatotoxicity and liver failure. Obtain baseline liver enzymes and monitor as clinically indicated.
- Fluid retention may require intervention.
- Decreases in haemoglobin.
- Pulmonary oedema in patients with pulmonary veno-occlusive disease (PVOD). If confirmed, discontinue treatment.
- Decreases in sperm count have been observed in patients taking ERAs.
Macitentan is contraindicated in the following:
- Pregnancy: If macitentan is used during pregnancy, apprise the patient of the potential hazard to the foetus.
- Women of childbearing potential who are not using reliable contraception
- Breastfeeding
- Patients with severe hepatic impairment (with or without cirrhosis)
- Baseline values of hepatic aminotransferases (aspartate aminotransferases (AST) and/or alanine aminotransferases (ALT) >3 × ULN).
Place of Macitentan in the Management of PAH
PAH is a chronic, incurable disease and the aim of therapy is to manage symptoms, ultimately prolonging patient survival and improving HRQoL. Treatment goals include
- enhancing functional capacity (as assessed by exercise endurance, such as the 6MWD);
- lowering mPAP;
- normalising cardiac output; and,
- reversing or preventing progression of disease.
Macitentan is a dual ETA/ETB ERA recently approved for the treatment of PAH. It is a derivative of bosentan, specifically designed to enhance tissue distribution and to optimise efficacy and safety.
Macitentan offers the following advantages:
- Pharmacological advantage
Exhibits prolonged receptor binding and increased tissue distribution in in vitro and in animal studies.
- Pharmacokinetic advantage
Has a long elimination half-life that allows for once-daily dosing.
Has similar or higher potency for the induction and inhibition of drug-metabolising enzymes (CYP3A4 and CYP2C19) and transporters (e.g. P-gp) than bosentan. However, because of the low plasma (~~0.63 lmol/L) and free (~~2.5 nmol/L) steady-state concentrations of macitentan at therapeutic dosages (10 mg/day), it is unlikely to inhibit these enzymes and transporters. In vitro and clinical studies have not shown any relevant effect of macitentan on CYP enzymes or transporters.
Macitentan, because of its increased lipophilicity, enters the liver by passive diffusion, resulting in low intrahepatic macitentan concentrations and limiting its interaction with NTCP and BSEP transport proteins involved in hepatic bile homeostasis. Multiple-dose administration of macitentan did not affect serum total bile salt levels to a significant extent.
- Proven clinical efficacy specific to long-term outcomes
The clinical efficacy of macitentan has been demonstrated in the randomised, placebo-controlled SERAPHIN study. This is the first large-scale, event-driven study of ERAs to assess the composite of morbidity and mortality as a primary endpoint, unlike previous ERA trials that had assessed short-term outcomes, such as 6MWD and WHO-FC.
SERAPHIN, which enrolled a broad PAH patient population, including patients with different aetiologies, WHO–FC and background therapy, showed that treatment with oral macitentan 10 mg once daily for a median of 115 weeks significantly reduced morbidity and mortality, as assessed by the primary composite endpoint, in patients with PAH (mostly of WHO-FC II or III), with the treatment effect largely attributable to a reduction in clinical worsening events.
Significant improvements with macitentan were also seen in other outcomes, including the rate of PAH-related death or hospitalisation, the 6MWD, WHO-FC, haemodynamic parameters, as well as HRQoL.
Moreover, subgroup analyses suggested a benefit of macitentan therapy across a broad range of subgroups, including in patients who were or were not receiving background PAH therapy.
SERAPHIN contributes both short- and long-term data on combination therapy with macitentan to the growing body of evidence for treatment of patients with PAH with multiple treatment modalities. Based on recent evidence from long-term randomised controlled trials and recommendations from the European Respiratory Society/European Society of Cardiology (ESC/ERS) guidelines for PAH, in the current treatment era, combination therapy regimens, such as those including macitentan and a PDE5i, are an important treatment option for patients with PAH.
Macitentan, thus, significantly reduces mortality and morbidity events like worsening due to PAH and risk and rates of hospitalisation. Current evidence suggests that macitentan is a useful treatment option for initial therapy in patients with WHO–FC II or III PAH.
Prescribing Information
For the use of a Cardiologist only
WARNING
EMBRYO-FOETAL TOXICITY
- Do not administer macitentan to a pregnant female because it may cause foetal harm.
- Females of reproductive potential: Exclude pregnancy before the start of treatment, monthly during treatment, and 1 month after stopping treatment. Prevent pregnancy during treatment and for 1 month after stopping treatment by using acceptable methods of contraception.
Composition
Each film coated tablet contains:
Macitentan………….10 mg
Colours: Titanium Dioxide IP
Dosage Form
Tablet
Pharmacology
Pharmacodynamics
Mechanism of Action
Macitentan is an endothelin receptor antagonist (ERA) that prevents the binding of ET-1 to both ETA and ETB receptors. Macitentan displays high affinity and sustained occupancy of the ET receptors in human pulmonary arterial smooth muscle cells. One of the metabolites of macitentan is also pharmacologically active at the ET receptors and is estimated to be about 20% as potent as the parent drug in vitro.
Pharmacodynamics Pulmonary Haemodynamics
Patients treated with macitentan 10 mg (N=57) achieved a median reduction of 37% (95% CI: 22 to 49) in pulmonary vascular resistance and an increase of 0.6 L/min/m2 (95% CI: 0.3 to 0.9) in the cardiac index compared with placebo (N=67).
Cardiac Electrophysiology
In a randomised, placebo-controlled, four-way crossover study with a positive control in healthy subjects, repeated doses of macitentan 10 and 30 mg (3 times the recommended dosage) had no significant effect on the QTc interval.
Pharmacokinetics
The pharmacokinetics of macitentan and its active metabolite have been studied primarily in healthy subjects. The pharmacokinetics of macitentan is dose-proportional over a range from 1 mg to 30 mg after once-daily administration. A cross-study comparison shows that the exposures to macitentan and its active metabolite in patients with PAH are similar to those observed in healthy subjects.
Absorption and Distribution
The maximum plasma concentration of macitentan is achieved about 8 hours after oral administration. The absolute bioavailability after oral administration is not known. In a study in healthy subjects, the exposure to macitentan and its active metabolite were unchanged after a high-fat breakfast. Macitentan may, therefore, be taken with or without food. Macitentan and its active metabolite are highly bound to plasma proteins (>99%), primarily to albumin and to a lesser extent to alpha-1-acid glycoprotein. The apparent volumes of distribution (Vss/F) of macitentan and its active metabolite were about 50 L and 40 L, respectively, in healthy subjects.
Metabolism and Elimination
Following oral administration, the apparent elimination half-lives of macitentan and its active metabolite are approximately 16 hours and 48 hours, respectively. Macitentan is metabolised primarily by oxidative depropylation of the sulphamide to form the pharmacologically active metabolite. This reaction is dependent on the cytochrome P450 (CYP) system, mainly CYP3A4 with a minor contribution of CYP2C19. At steady state in pulmonary arterial hypertension (PAH) patients, the systemic exposure to the active metabolite is 3 times the exposure to macitentan and is expected to contribute approximately 40% of the total pharmacologic activity. In a study in healthy subjects with radiolabelled macitentan, approximately 50% of radioactive drug material was eliminated in urine but none was in the form of unchanged drug or the active metabolite. About 24% of the radioactive drug material was recovered from the faeces.
Special Populations
There are no clinically relevant effects of age, sex, or race on the pharmacokinetics of macitentan and its active metabolite.
Renal Impairment
Exposure to macitentan and its active metabolite in patients with severe renal impairment (creatinine clearance [CrCl] 15 to 29 mL/min) compared to healthy subjects was increased by 30% and 60%, respectively. This increase is not considered clinically relevant.
Hepatic Impairment
Exposure to macitentan was decreased by 21%, 34% and 6%, and exposure to the active metabolite was decreased by 20%, 25% and 25% in subjects with mild, moderate, or severe hepatic impairment (Child-Pugh Class A, B, and C), respectively. This decrease is not considered clinically relevant.
Indications
OPSUTAN Tablets are indicated for the treatment of PAH (WHO Group I) to delay disease progression.
Disease progression included death, initiation of intravenous (IV) or subcutaneous prostanoids, or clinical worsening of PAH (decreased 6-minute walk distance, worsened PAH symptoms and need for additional PAH treatment).
Macitentan also reduces hospitalisation for PAH.
Dosage and Administration
The recommended dosage of OPSUTAN Tablets is 10 mg once daily for oral administration. Doses higher than 10 mg once daily have not been studied in patients with PAH and are not recommended.
OPSUTAN Tablets should be taken every day at about the same time. If the patient misses a dose of OPSUTAN Tablets, the patient should be told to take it as soon as possible and then take the next dose at the regularly scheduled time. The patient should be told not to take two doses at the same time if a dose has been missed.
Pregnancy Testing in Females of Reproductive Potential
Initiate treatment with OPSUTAN Tablets in females of reproductive potential only after a negative pregnancy test. Obtain a monthly pregnancy test during treatment.
Contraindications
- Hypersensitivity to the active substance or to any of the excipients
- Pregnancy: Macitentan is contraindicated in females who are pregnant. If macitentan is used during pregnancy, apprise the patient of the potential hazard to the foetus.
- Women of childbearing potential who are not using reliable contraception
- Breastfeeding
- Patients with severe hepatic impairment (with or without cirrhosis)
- Baseline values of hepatic aminotransferases (aspartate aminotransferases (AST) and/or alanine aminotransferases (ALT) >3 × ULN)
Warnings and Precautions
Embryo-Foetal Toxicity
Macitentan may cause foetal harm when administered during pregnancy and is contraindicated for use in females who are pregnant. In females of reproductive potential, exclude pregnancy prior to initiation of therapy, ensure use of acceptable contraceptive methods and obtain monthly pregnancy tests.
Hepatotoxicity
ERAs have caused elevations of aminotransferases, hepatotoxicity, and liver failure. Macitentan is not to be initiated in patients with severe hepatic impairment or elevated aminotransferases (>3 × ULN), and is not recommended in patients with moderate hepatic impairment. Liver enzyme tests should be obtained prior to initiation of macitentan.
Advise patients to report symptoms suggesting hepatic injury (nausea, vomiting, right upper quadrant pain, fatigue, anorexia, jaundice, dark urine, fever, or itching). If clinically relevant aminotransferase elevations occur, or if elevations are accompanied by an increase in bilirubin >2 x ULN, or by clinical symptoms of hepatotoxicity, discontinue macitentan.
Consider re-initiation of macitentan when hepatic enzyme levels normalise in patients who have not experienced clinical symptoms of hepatotoxicity.
Fluid Retention
Peripheral oedema and fluid retention are known clinical consequences of PAH and known effects of ERAs. Patients with underlying left ventricular dysfunction may be at particular risk for developing significant fluid retention after initiation of ERA treatment.
Postmarketing cases of oedema and fluid retention occurring within weeks of starting macitentan, some requiring intervention with a diuretic or hospitalisation for decompensated heart failure, have been reported.
Monitor for signs of fluid retention after macitentan initiation. If clinically significant fluid retention develops, evaluate the patient to determine the cause, such as macitentan or underlying heart failure, and the possible need to discontinue macitentan.
Haemoglobin Decrease
As with other ERAs, treatment with macitentan has been associated with a decrease in haemoglobin concentration. Cases of anaemia requiring blood cell transfusion have been reported with macitentan and other ERAs. Initiation of macitentan is not recommended in patients with severe anaemia. It is recommended that haemoglobin concentrations be measured prior to initiation of treatment and tests repeated during treatment as clinically indicated.
Pulmonary Oedema with Pulmonary Veno-occlusive Disease (PVOD)
Cases of pulmonary oedema have been reported with vasodilators (mainly prostacyclins) when used in patients with PVOD. Should signs of pulmonary oedema occur, consider the possibility of associated PVOD. If confirmed, discontinue macitentan.
Decreased Sperm Counts
Other ERAs have caused adverse effects on spermatogenesis. Male patients should be counselled about the potential effects on fertility.
Excipients
Macitentan tablets contain lactose. Patients with rare hereditary problems of galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Macitentan tablets contain lecithin derived from soya. If a patient is hypersensitive to soya, macitentan must not be used.
Drug Interactions
In vitro Studies
The CYP450 enzymes, CYP3A4, CYP2C8, CYP2C9 and CYP2C19, are involved in the metabolism of macitentan and formation of its metabolites. Macitentan and its active metabolite do not have clinically relevant inhibitory or inducing effects on CYP450 enzymes.
Macitentan and its active metabolite are not inhibitors of hepatic or renal uptake transporters at clinically relevant concentrations, including the organic anion-transporting polypeptides (OATP1B1 and OATP1B3).
Macitentan and its active metabolite are not relevant substrates of OATP1B1 and OATP1B3, but enter the liver by passive diffusion.
Macitentan and its active metabolite are not inhibitors of hepatic or renal efflux pumps at clinically relevant concentrations, including the multidrug-resistant protein (P-gp, MDR-1) and multidrug and toxin extrusion transporters (MATE1 and MATE2-K). Macitentan inhibits the breast cancer resistance protein (BCRP) at clinically relevant intestinal concentrations.
Macitentan is not a substrate for P-gp/MDR-1. At clinically relevant concentrations, macitentan and its active metabolite do not interact with proteins involved in hepatic bile salt transport, i.e. the bile salt export pump (BSEP) and the sodium-dependent taurocholate co-transporting polypeptide (NTCP).
In vivo Studies
Strong CYP3A4 Inducers
Strong inducers of CYP3A4 (such as rifampin, St. John’s wort, carbamazepine and phenytoin) significantly reduce macitentan exposure. Concomitant use of macitentan with strong CYP3A4 inducers should be avoided.
Strong CYP3A4 Inhibitors
Concomitant use of strong CYP3A4 inhibitors (e.g. itraconazole, ketoconazole, voriconazole, clarithromycin, telithromycin, nefazodone, ritonavir and saquinavir) approximately double macitentan exposure. Many HIV drugs such as ritonavir are strong inhibitors of CYP3A4. Avoid concomitant use of macitentan with strong CYP3A4 inhibitors. Use other PAH treatment options when strong CYP3A4 inhibitors are needed as part of HIV treatment.
Warfarin
Macitentan, given as multiple doses of 10 mg once daily, had no effect on exposure to S-warfarin (CYP2C9 substrate) or R-warfarin (CYP3A4 substrate) after a single dose of 25 mg warfarin. The pharmacodynamic effect of warfarin on the International Normalised Ratio (INR) was not affected by macitentan. The pharmacokinetics of macitentan and its active metabolite were not affected by warfarin.
Sildenafil
At steady state, the exposure to sildenafil 20 mg t.i.d. was increased by 15% during concomitant administration of macitentan 10 mg once daily. Sildenafil, a CYP3A4 substrate, did not affect the pharmacokinetics of macitentan, while there was a 15% reduction in the exposure to the active metabolite of macitentan. These changes are not considered clinically relevant.
Cyclosporine A
Concomitant treatment with cyclosporine A 100 mg b.i.d., a combined CYP3A4 and OATP inhibitor, did not alter the steady-state exposure to macitentan and its active metabolite to a clinically relevant extent.
Hormonal Contraceptives
Macitentan 10 mg once daily did not affect the pharmacokinetics of an oral contraceptive (norethisterone 1 mg and ethinyl oestradiol 35 μg).
Use in Special Populations
Renal Impairment
Based on pharmacokinetic data, no dose adjustment is required in patients with renal impairment. There is no clinical experience with the use of macitentan in PAH patients with severe renal impairment.
The use of macitentan is not recommended in patients undergoing dialysis. Caution is recommended in this population. Patients with renal impairment may run a higher risk of experiencing hypotension and anaemia during treatment with macitentan. Therefore, monitoring of blood pressure and haemoglobin should be considered.
Hepatic Impairment
Based on pharmacokinetic data, no dose adjustment is required in patients with mild, moderate or severe hepatic impairment. However, there is no clinical experience with the use of macitentan in PAH patients with moderate or severe hepatic impairment. Macitentan must not be initiated in patients with severe hepatic impairment, or clinically significant elevated hepatic aminotransferases (greater than 3 times the upper limit of normal (>3 × ULN). Liver enzyme tests should be obtained prior to initiation of macitentan. If sustained, unexplained, clinically relevant aminotransferase elevations occur, or if elevations are accompanied by an increase in bilirubin >2 × ULN, or by clinical symptoms of liver injury (e.g. jaundice), macitentan treatment should be discontinued.
Pregnancy
Pregnancy Category X
Risk Summary
Macitentan may cause foetal harm when administered to a pregnant woman and is contraindicated during pregnancy. Macitentan was teratogenic in rabbits and rats at all doses tested. A no-effect dose was not established in either species. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, advise the patient of the potential hazard to a foetus.
Lactation
It is not known whether macitentan is present in human milk. Macitentan and its metabolites were present in the milk of lactating rats. Because many drugs are present in human milk and because of the potential for serious adverse reactions from macitentan in nursing infants, nursing mothers should discontinue nursing or discontinue macitentan.
Paediatric Use
The safety and efficacy of macitentan in children has not been established.
Geriatric Use
No dose adjustment is required in patients over the age of 65 years. There is limited clinical experience in patients over the age of 75 years. Therefore, macitentan should be used with caution in this population.
Females and Males of Reproductive Potential
Females: Pregnancy Testing
Female patients of reproductive potential must have a negative pregnancy test prior to starting treatment with macitentan and monthly pregnancy tests during treatment with macitentan. Advise patients to contact their healthcare provider if they become pregnant or suspect they may be pregnant. Perform a pregnancy test if pregnancy is suspected for any reason. For positive pregnancy tests, counsel patients on the potential risk to the foetus.
Contraception
Female patients of reproductive potential must use acceptable methods of contraception during treatment with macitentan and for 1 month after treatment with macitentan. Patients may choose one highly effective form of contraception (intrauterine devices [IUD], contraceptive implants or tubal sterilisation) or a combination of methods (hormone method with a barrier method or two barrier methods). If a partner’s vasectomy is the chosen method of contraception, a hormone or barrier method must be used along with this method. Counsel patients on pregnancy planning and prevention, including emergency contraception, or designate counselling by another healthcare provider trained in contraceptive counselling.
Males: Testicular Effects
Like other ERAs, macitentan may have an adverse effect on spermatogenesis.
Undesirable Effects
Clinically significant adverse reactions that appear in other sections of the labelling include the following:
- Embryo-Foetal Toxicity
- Hepatotoxicity
- Fluid Retention
- Decrease in Haemoglobin
Clinical Trial Experience
Tabulated List of Adverse Reactions
Adverse reactions associated with macitentan obtained from the clinical study are tabulated below.
Frequencies are defined as very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000).
|
System organ class
|
Frequency
|
Adverse reaction
|
|
Infections and infestations
|
Very common
|
Nasopharyngitis
|
|
|
Very common |
Bronchitis
|
|
|
Common
|
Pharyngitis
|
|
|
Common
|
Influenza
|
|
|
Common
|
Urinary tract infection
|
|
Blood and lymphatic system disorders |
Very common
|
Influenza
|
|
Immune system disorders
|
Uncommon
|
Hypersensitivity reactions (e.g. angio-oedema, pruritus, rash)
|
|
Nervous system disorders
|
Very common
|
Headache
|
|
Vascular disorders
|
Common
|
Hypotension
|
|
Respiratory, thoracic and mediastinal disorders
|
Common
|
Nasal congestion
|
|
General disorders and administration site conditions
|
Very common
|
Oedema, fluid retention
|
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of macitentan. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Immune System Disorders: Hypersensitivity reactions (angio-oedema, pruritus and rash).
- Respiratory, Thoracic and Mediastinal Disorders: Nasal congestion.
- Gastrointestinal Disorders: Elevations of liver aminotransferases (ALT, AST) and liver injury have been reported with macitentan use; in most cases, alternative causes could be identified (heart failure, hepatic congestion, autoimmune hepatitis).
- ERAs have been associated with elevations of aminotransferases, hepatotoxicity, and cases of liver failure.
- General Disorders and Administration Site Conditions: Oedema/fluid retention. Cases of oedema and fluid retention occurred within weeks of starting macitentan, some requiring intervention with a diuretic, fluid management or hospitalisation for decompensated heart failure.
- Cardiac Disorders: Symptomatic hypotension.
If you experience any side-effects, talk to your doctor or pharmacist or write to drugsafety@cipla.com. You can also report side-effects directly via the National Pharmacovigilance Programme of India by calling on 1800 180 3024.
By reporting side-effects, you can help provide more information on the safety of this product.
Overdosage
Macitentan has been administered as a single dose of up to and including 600 mg to healthy subjects (60 times the approved dosage). Adverse reactions of headache, nausea and vomiting were observed. In the event of an overdose, standard supportive measures should be taken, as required. Dialysis is unlikely to be effective because macitentan is highly protein-bound.
Storage and Handling Instructions
Store below 30°C.
Packaging Information
OPSUTAN Tablets: Blister pack of 10 tablets
February 2018
References
- White JR, Lannan KL, Phipps RP. Drug discovery in pulmonary arterial hypertension: attacking the enigmatic root of a deadly weed. Drug Discov Today. 2014 Aug;19(8):1226-9.
- Dhillon, S. Macitentan: A Review of Its Use in Patients with Pulmonary Arterial Hypertension, Drugs (2014) 74:1495–1507.
- Hong, IS, Coe HV, Catanzaro LM. Macitentan for the Treatment of Pulmonary Arterial Hypertension, Ann Pharmacother. 2014 Apr;48(4):538-47.
- Channick RN, Delcroix M, Ghofrani HA. Effect of macitentan on hospitalizations: results from the SERAPHIN trial. JACC Heart Fail. 2015 Jan;3(1):1-8.
- Galie, N, Jansa, P, Pulido, T et al. SERAPHIN haemodynamic substudy: the effect of the dual endothelin receptor antagonist macitentan on haemodynamic parameters and NT-proBNP levels and their association with disease progression in patients with pulmonary arterial hypertension, European Heart Journal (2017) 38, 1147–1155.
- Burger CD, Long PK, Shah MR, et al. Characterization of first-time hospitalizations in patients with newly diagnosed pulmonary arterial hypertension in the REVEAL registry. Chest 2014;146:1263–73.
- Thenappan T, Ryan JJ, Archer SL. Evolving epidemiology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012 Oct 15;186(8):707-9.
- Bhogal S, Mukherjee D, Banerjee S. Current Trends and Future Perspectives in the Treatment of Pulmonary Arterial Hypertension. Curr Probl Cardiol. 2017 Oct 31.
- PubChem, Open Chemistry Database https://pubchem.ncbi.nlm.nih.gov/compound/Macitentan#section=Top
- Pulido T, Adzerikho I, Channick RN et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013 Aug 29;369(9):809-1.
- Mehta, S, Sastry, BKS, Souza, R et al. Macitentan Improves Health-Related Quality of Life for Patients With Pulmonary Arterial Hypertension Results From the Randomized Controlled SERAPHIN Trial CHEST 2017; 151(1):106-118.
- Jansa P, Pulido T. Macitentan in Pulmonary Arterial Hypertension: A Focus on Combination Therapy in the SERAPHIN Trial. Am J Cardiovasc Drugs. 2018 Feb;18(1):1-11.
- Ghofrani HA, Simonneau G, D'Armini AM. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, Phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med. 2017 Oct;5(10):785-794
- Blok IM, van Riel ACMJ1, van Dijk APJ et al. From bosentan to macitentan for pulmonary arterial hypertension and adult congenital heart disease: Further improvement? Int J Cardiol. 2017 Jan 15;227:51-52.
- Cyrus A Kholdani,Wassim H Fares,Terence K Trow. Macitentan for the treatment of pulmonary arterial hypertension. Vasc Health and Risk Manag 2014:10 665–673.


.jpg?updated=20230105104134)
.jpg?updated=20230105104240)
.jpg?updated=20230105104344)
.jpg?updated=20230105104445)
.jpg?updated=20230105104840)
.jpg?updated=20230105104948)
.jpg?updated=20230105105154)
.jpg?updated=20230105105309)
.jpg?updated=20230105105819)
.jpg?updated=20230105105930)