Mexiletine FAQ Booklet
What are the specific types of ventricular tachycardia where mexiletine is the most effective?
Mexiletine is a sodium channel blocker classified as a Class 1B antiarrhythmic drug. It affects phase 0 of the cardiac myocyte action potential, inhibiting the inward sodium current. As a class 1B antiarrhythmic, mexiletine binds best to sodium channels in the depolarized state. Class 1 antiarrhythmic drugs exhibit use dependence whereby they block sodium channels best when these channels are in use, or specifically when the sodium channel is in an open or an inactive state. Cardiac myocyte sodium channels are in these states more often at faster heart rates, allowing more drug to bind at these higher rates. Thus, use dependence means more binding at faster heart rates, and its clinical relevance is that tachycardia can be dangerous as there is an increased risk of adverse effects and toxicity at these faster rates. Mexiletine exhibits use dependence by rapidly unbinding when the myocytes re-polarize and no longer remain in the depolarized state, or when the sodium channels are in a resting state (not in use).1 Mexiletine has more pronounced effects on diseased (e.g. ischemic) than healthy tissue. It has more prominent effects on ventricular muscle at higher extracellular potassium concentrations. It is capable of preventing re-entrant-type arrhythmias in ischemic zone cells. Thus, it is effective for ventricular arrhythmias occurring in depolarised fibres, e.g. in acute myocardial ischaemia.2 Mexiletine shortens refractory periods and conduction times in acutely ischemic tissues, particularly in Purkinje cells where these are most prolonged. This results in uniformity of conduction and refractoriness in the ventricular myocardium. This effect is postulated to reduce the possibility of re- entrant arrhythmias. In combination with other Type IA antiarrhythmic agents, mexiletine produces a synergistic lengthening of the ventricular effective refractory period and effective refractory period to action potential duration ratio (ERP/APD), without significant effects on ventricular conduction time and excitability threshold.3 In clinical evidence, mexiletine has demonstrated efficacy in the suppression of ventricular arrhythmias in patients with ischemic and other forms of structural heart disease and in resistant ventricular tachycardia (VT) when combined with other antiarrhythmic agents.3 Mexiletine is an effective therapy in the treatment of premature ventricular contractions (PVCs) with or without associated acute myocardial infarction.4 In patients with drug-refractory inducible VT, mexiletine is usually ineffective as monotherapy. But when combined with other antiarrhythmic agents, mexiletine showed good response in a significantly higher percentage of patients with this difficult arrhythmia.4 In patients with sodium channel-mediated type 3 long QT syndrome (LQT3), mexiletine is beneficial in imparting a significant reduction of arrhythmic events due to its potential as sodium channel blocker. In clinical evidence, mexiletine significantly reduced the QTc in these patients both in the short- and long-term follow-up.5 Mexiletine when added to amiodarone is efficacious in patients with implantable cardioverter‑defibrillator (ICD) exhibiting amiodarone-refractory ventricular arrhythmia (VA) but not eligible for catheter ablation. This combination can significantly decrease the number of total ICD shocks, increase appropriate anti-tachycardia pacing and reduce the amiodarone dose during the follow-up.6 Mexiletine is as effective as procainamide in the prevention of ventricular arrhythmias after acute myocardial infarction but is superior in terms of advantages like less frequent administration and lower toxicity.7
What are the guideline recommendations for clinical use of mexiletine in ventricular arrhythmias?
Mexiletine is recommended by the international guidelines for the management of ventricular arrhythmias (VA). 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death recommends mexiletine as a therapeutic option for prevention or treatment of ventricular tachyarrhythmias or ventricular fibrillation.8
Recommendations by 2014 EHRA/HRS/APHRS expert consensus on VAs: 9
- Mexiletine is amongst the AADs in patients suffering from symptomatic non-sustained VAs on an adequately dosed beta- blocker or a non-dihydropyridine calcium channel antagonist to improve symptoms associated with arrhythmia episodes (Class IIb LOE C).
- Mexiletine features amongst the recommended AADs for patients with structural heart disease (SHD) and recurrent sustained monomorphic VT (SMVT) in addition to ICD and ablation (Class IIa LOE B).
What are the salient features of mexiletine as an AAD?
Mexiletine has several salient features as an effective anti-arrhythmic drug.3 Following are the favorable pharmacokinetic features of mexiletine:3
- Half-life of 9-12 hours
- Almost complete absorption
- Minimal first‑ pass hepatic biotransformation
As compared to other antiarrhythmics, mexiletine has several advantages which are enlisted below:
- Mexiletine is a Class 1B antiarrhythmic and oral congener of lidocaine3
- Pronounced action on ischemic myocardium3
- No significant haemodynamic effects2
- Does not adversely affect already compromised left ventricular function in patients with cardiomyopathy or acute myocardial infarction2
- Relatively safe electrophysiological effects: Mexiletine does not prolong QRS or QT (QTc) intervals and has a low propensity for proarrhythmic effects2
- Reversible adverse effects: Side‑effects of mexiletine are mostly non‑serious and can be controlled by dosage reductions or administration with food2
- Can be safely administered in combination with other antiarrhythmic drugs such as amiodarone or beta blockers to improve efficacy and tolerability2
What is the dosage regimen of mexiletine in ventricular arrhythmias?
Mexiletine has a narrow therapeutic index and its efficacy is limited at plasma concentrations below 0.5 mg/L while at concentrations above 2.0 mg/L the risk of intolerable side effects is high.2 The dosage of mexiletine must be individualized on the basis, of response and tolerance, both of which are dose-related. As with any antiarrhythmic drug, clinical and electrocardiographic evaluation (including Holter monitoring if necessary for evaluation) are needed to determine whether the desired antiarrhythmic effect has been obtained and to guide titration and dose adjustment.10 The recommended oral dosage of mexiletine is 150–300 mg every 8 hour or every 12 hours and peak concentrations occurs in 1 to 3 hours. An initial dose of 150 to 200 mg every 8 hour is gradually increased and is associated with a lower frequency of side effects.8,11 A minimum of 2 to 3 days between dose adjustments is recommended. Dose may be adjusted in 50 or 100 mg increments up or down. Some patients responding to mexiletine may be transferred to a 12‑hour dosage schedule to improve convenience and compliance. When rapid control of arrhythmias is required, a 400 mg loading dose of mexiletine orally followed by 200 mg dose in 8 hours is the ideal regimen.10 Caution is necessary when lidocaine is administered concurrently due to possibility additive effects. The use of lidocaine should be discontinued 1 to 2 hours before the initiation of mexiletine therapy.11 Administration with food or antacid is recommended.10
Which set of patients would need dosage adjustment for mexiletine?
Hepatic Dysfunction: The metabolism of mexiletine is predominantly hepatic and is extensive and therefore patients with hepatic disease experience pronounced delay in elimination.2,3
Acute Liver Injury: In post‑marketing experience, abnormal liver function tests have been reported, some in the first few weeks of therapy with mexiletine. Most of these have been observed in the setting of congestive heart failure or ischemia and their relationship to mexiletine has not been established.10 Due to interaction with drugs which induce hepatic mixed function oxidase enzymes (e.g., diphenylhydantoin and rifampin) mexiletine elimination may significantly reduce, resulting in unexpectedly low drug levels.3 Thus, particular care is taken during dosage titration as these patients.2 Patients with severe liver disease, however, may require lower doses and must be monitored closely. Similarly, marked right‑sided congestive heart failure can reduce hepatic metabolism and reduce the needed dose.10 In patients with cirrhosis, reduction in maintenance dosage of mexiletine by one-third or one-quarter is recommended.2
Congestive Heart Failure: As half‑life of mexiletine is prolonged in patients with congestive heart failure; dose reduction is recommended in such patients.11
Renal Disease: Patients with renal failure will require usual doses of mexiletine.10
Pregnancy & Lactation: Pregnancy Category C - Mexiletine should be used during pregnancy only if the potential benefits justifies the potential risk to the fetus. Mexiletine appears in human milk in concentrations similar to those observed in plasma. Therefore, if the use of mexiletine is deemed essential, an alternative method of infant feeding should be considered.10
Pediatric Use: Safety and effectiveness in the pediatric population have not been established.10
What is the place of mexiletine in therapy for ventricular arrhythmias?
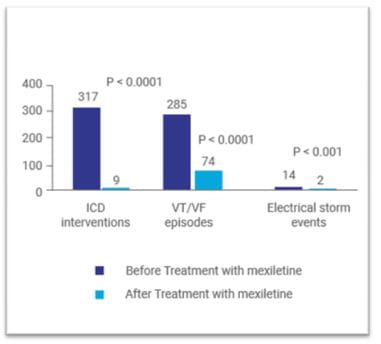
Mexiletine is approved in the treatment of documented ventricular arrhythmias (VAs).12 Mexiletine is moderately effective in selected patients with recurrent ventricular tachycardia (VT); however, in combination with other antiarrhythmic agents is more often effective in drug-refractory patients.6 In a study by Waleffe and colleagues, oral mexiletine was combined with amiodarone (each 600 mg daily) in patients with recurrent refractory VT. Total suppression of the tachycardia episodes within 3 days after initiation of the therapy was reported in 33% of the patients. In 66% of the patients, no arrhythmia could be elicited by programmed electrical stimulation of the heart on the 7th day of treatment. This study thus highlighted that the combination of a class I (mexiletine) with a class III (amiodarone) agent can be a viable option for the treatment of refractory VAs.13 In a study by Ravid and colleagues, complementary effect of low-dose mexiletine and metoprolol, as compared with either drug alone was assessed in patients with frequent VAs including those with non-sustained VT. This study reported that the combination therapy was effective in reducing VAs in 80% of the patients while only 10% on metoprolol alone and 40% on mexiletine alone achieved this result. VT was abolished in 71% of the patients. Further, combination therapy was well tolerated without proarrhythmia or precipitation of congestive heart failure. Left ventricular systolic function was unchanged with the regimen when measured by repeat echocardiograms.14 Some patients with implantable cardioverter‑defibrillator (ICD) who have amiodarone‑refractory VA may not be eligible for catheter ablation. A recent study by Madadi and colleagues assessed the efficacy of mexiletine in combination with amiodarone in the reduction of VA in this group of patients. This study reported a significant decrease in the number of total ICD shock and significant increase in appropriate antitachycardia pacing during follow-up after initiating mexiletine. Mexiletine therapy also significantly ICD interventions VT/VF episodes Electrical storm events reduced the amiodarone dose during the follow-up. No mortality was observed in the present cohort during the study period. Another advantage of this combination was a lack of significant effect on QRS width, QTc interval, and PR interval in the patients (all P > 0.05).6 Another study by Sobiech and associates evaluated the efficacy and tolerability of mexiletine in patients with recurrent ventricular tachyarrhythmias and/or electrical storm events, in whom standard treatment strategies (beta-adrenergic drugs plus amiodarone or radiofrequency ablation) failed to prevent ventricular tachyarrhythmia. In this study, all except one patient had an ICD implanted. Mexiletine treatment was initiated in few patients after the episode of electrical storm (3 or more separate episodes of VT/VF demanding ICD discharge in a period of 24 hours). In others, the drug was initiated after numerous recurrent VT/VF episodes that did not fulfil the criteria for electrical storm. This study reported that treatment with mexiletine significantly reduced the number of electrical storm events, VT/VF episodes and ICD interventions in comparison with a matched period before initiation of treatment (Figure 1). Sufficient tolerability of mexiletine was observed in 82% of the patients and only 18% showed severe side effects requiring discontinuation of the therapy.15
A study by Gao D and associates reported that mexiletine, when added to amiodarone in case of amiodarone inefficacy, reduced VT/fibrillation events despite appropriate therapies in patients with an ICD.16 Mazzanti and associates demonstrated the effectiveness of mexiletine in preventing arrhythmic events (arrhythmic syncope, aborted cardiac arrest, or sudden cardiac death) in long QT syndrome type 3 (LQT3) patients. In the study the patients received oral mexiletine therapy for at least 3 years. Mexiletine significantly shortened QTc (by 63 ± 6 ms; p < 0.0001) and reduced the percentage of patients with arrhythmic events (from 22% to 3%; p = 0.031), the mean number of arrhythmic events per patient (from 0.43 ± 0.17 to 0.03 ± 0.03; p = 0.027), and the annual rate of arrhythmic events (from 10.3% to 0.7%; p = 0.0097). The study showcased that mexiletine is an efficacious therapeutic strategy, as it can shorten QTc interval and cause major reduction of life- threatening arrhythmic events in LQT3 patients.17 Overall, mexiletine can be preferred as an add‑on therapy in recurrent/refractory VT or electrical storm events associated with ischemic cardiomyopathy. The drug can be combined with other antiarrhythmic drugs such as amiodarone or beta blockers as well as ICD implantation. The drug is particularly useful in the management of ventricular arrhythmias in long QT syndrome subtype 3.2,6
Does mexiletine have a role in patients with implantable cardioverter defibrillators?
Patients with implantable cardioverter defibrillators (ICDs) are at a high risk of developing recurrent ventricular tachycardia or ventricular fibrillation (VT/VF) episodes. Further, these patients experience inappropriate ICD shocks which is associated with worse clinical outcome. Electrical storm events and their recurrences on the other hand poses a life-threatening situation.15 A study by Sobiech et al. evaluated the efficacy and tolerability of mexiletine in patients with recurrent ventricular tachyarrhythmias and/or electrical storm events, in whom standard treatment strategies failed to prevent ventricular tachyarrhythmia. This study reported that treatment with mexiletine significantly reduced the number of electrical storm events, VT/VF episodes and ICD interventions, in comparison with a matched period before initiation of treatment. Mexiletine was safe and well tolerated in these patients.15 A study by Madadi S and colleagues assessed the efficacy of mexiletine in combination with amiodarone in the reduction of ventricular arrhythmia (VA) in implantable cardioverter‑defibrillator (ICD) in patients with amiodarone-refractory of VA. This study reported a significant decrease in the number of total ICD shock and increase in appropriate antitachycardia pacing and reduction in the amiodarone dose during follow-up after initiating mexiletine. There were no cases of mortality reported during the study period.6 Similar results were reported in a study by Gao D and colleagues where mexiletine was used as an adjunctive therapy to amiodarone. In this study, the combination reduced the frequency of ventricular tachyarrhythmia events, VT/VF events (median 2 vs. 12 events, P = 0.001) and shocks (median 0 vs. 2 events, P = 0.003) in the first 3 months of treatment. Further, the combination also reduced the requirement of appropriate therapies in patients with an implantable cardioverter defibrillator.16
What are the drug interactions reported with mexiletine?
Mexiletine is a substrate for the metabolic pathways involving CYP2D6 and CYP1A2 enzymes and hence inhibition or induction of either of these enzymes would be expected to alter mexiletine plasma concentrations.10 Drugs like phenobarbital, primidone, rifampin or phenytoin which induce the hepatic enzymes may influence the rate of metabolism and excretion of mexiletine. Thus, the dosage of mexiletine must be increased when it is administered concomitantly with any of these agents to maintain therapeutic blood levels of the drug.2,11 Cimetidine, isoniazid, chloramphenicol, dicoumarol, disulfiram and methylphenidate have been shown to decrease the clearance of mexiletine and prolong its half- life.9 Mexiletine can reduce caffeine and theophylline elimination and this may be accompanied by aggravation of arrhythmia. As changes in urine pH may affect renal clearance of mexiletine, concomitant administration of alkalinizing agents may increase the half-life of mexiletine.3 Concomitant administration of mexiletine and theophylline can lead to theophylline toxicity and enhanced proarrhythmic effects as mexiletine appears to reduce the rate of clearance of methylxanthines.2 Cigarette smoking may alter the kinetics of mexiletine by decreasing its elimination half-life.3 Absorption of mexiletine is affected due to delayed gastric emptying, which may occur following myocardial infarction or after narcotic administration and when drugs like antacids, cimetidine and atropine are administered. Absorption of mexiletine is enhanced with administration of metoclopramide, which promotes gastric emptying.3 Recently, few case reports have raised the possibility of interaction of mexiletine with sacubitril/valsartan resulting in proarrhythmic effects. Extreme caution and careful monitoring for arrhythmias should be exercised in patients receiving sacubitril/valsartan and mexiletine.18
Does mexiletine exert any interaction with beta blockers?
Mexiletine can be used in combination with a beta blocker for the management of ventricular arrhythmia as such a strategy imparts independent advantages. Beta blockers can increase the threshold to malignant ventricular arrhythmia, antagonize the catecholamine-induced reversal of the antiarrhythmic effects of membrane active drugs and reduce mortality and sudden death following myocardial infarction. On the other hand, mexiletine has a favorable hemodynamic profile and low incidence of proarrhythmia.14 In a study by Ravid and colleagues, the low-dose mexiletine and metoprolol, effectively reduced ventricular arrhythmias in 80% of the patients while monotherapies with metoprolol and mexiletine showed reductions in 10 and 40% of patients respectively. Complete abolition of ventricular tachycardia (VT) was reported in 71% of the patients. Further, combination therapy also significantly reduced number of couplets and total premature ventricular beats. The combination therapy was well-tolerated without proarrhythmia or precipitation of congestive heart failure.14 In another study by Kobayashi A et al, a combination of mexiletine (300 mg/day) and a low dose of propranolol (30 mg/day) significantly decreased the frequency of ventricular premature beats (VPBs) in 58% patients and lowered the severity of the grade in 30.8% patients who did not respond effectively to individual components. A change from combination therapy to propranolol was assessed as "undesirable" in 61.5% cases.19
What are the commonly observed adverse events with mexiletine?
Mexiletine is well-tolerated and commonly produces reversible gastrointestinal and nervous system adverse reactions. These include upper gastrointestinal distress, lightheadedness, tremor and coordination difficulties. These reactions are generally not serious, are dose-related and reversible with a reduction in dosage, by taking the drug with food or antacid or by therapy discontinuation.10 Adverse effects reported with mexiletine can be categorized as cardiovascular and non-cardiovascular.11
Cardiovascular adverse effects:11
- Worsening of congestive heart failure: < 3%
- Proarrhythmic effects: 10%
- Heart block: 1%
Noncardiovascular adverse effects:
- Overall Prevalence: 47 to 65%11
- Prevalence of neurologic side effects: 25%3
- Prevalence of gastrointestinal side effects: 39%11
The commonly encountered neurological side‑effects include fine intention hand tremor, lightheadedness, dizziness, ataxia, paresthesias or tingling of the extremities, throat numbness or oral cold sensation, blurred vision, nervous agitation, convulsions and even a peculiar perception of a menthol taste.11 In post-marketing experience, there have been isolated, spontaneous reports of pulmonary changes including pulmonary infiltration and pulmonary fibrosis during mexiletine therapy with or without other drugs or diseases that are known to produce pulmonary toxicity. A causal relationship to mexiletine therapy has not been established. In addition, there have been isolated reports of drowsiness, nystagmus, ataxia, dyspepsia, hypersensitivity reaction and exacerbation of congestive heart failure in patients with pre-existing compromised ventricular function. There have been rare reports of pancreatitis associated with mexiletine treatment.10 In less than 1% of patients, rare side effects like abnormal results of liver function tests, positive antinuclear antibodies (lupus syndrome), thrombocytopenia, leukopenia (neutropenia and agranulocytosis) and impotence have been reported.11
Does mexiletine have a negative impact on hemodynamic parameters?
Mexiletine therapy is associated with insignificant hemodynamic and minimal negative inotropic effects in patients with normal or near-normal left ventricular function as well as those with depressed myocardial function.2 In clinical studies, mexiletine showed no effect on mean hemodynamic parameters in patients with ventricular arrhythmias and ischemic heart disease and/or congestive heart failure. Mexiletine showed no influence on peak heart rate or blood pressure during exercise in patients with ventricular arrhythmias or impaired left ventricular function.2 In patients with left ventricular ejection fraction <50%, oral mexiletine was not associated with any significant change in left ventricular ejection fraction or right ventricular ejection fraction thus confirming the minimal adverse hemodynamic effects associated with the drug.20
What are the electrophysiological and long term adverse effects of mexiletine in comparison to other AADs?
Mexiletine has minimal effects on sinus rate or atrial refractoriness and intracardiac conduction in patients without sinus node dysfunction or conduction abnormalities. No consistent atrioventricular nodal or His-Purkinje conduction effects are noted with mexiletine. Atrioventricular (AV) nodal block is rare, and the PR, QRS, or QT intervals remain unchanged with mexiletine.3,11 However, mexiletine is contraindicated in cases of pre-existing second- or third-degree AV block.21 As compared with other antiarrhythmic agents drugs (AADs), mexiletine is relatively safe as its use is not associated with SA depression, AV nodal block or QT prolongation (Table).22 Long term administration of mexiletine is not associated with significant changes in the electrocardiogram (ECG}. The QRS duration remains unchanged while changes in mean PR and QTc intervals are comparatively smaller with mexiletine even after 3 months of administration.2
|
Drug |
Effect on SA nodal rate |
Effect on AV nodal refractory period
|
PR interval |
QRS duration |
QT interval |
|
Amiodarone |
↓↓ ↓↓ |
↑↑ |
Variable |
↑ |
↑↑↑↑ |
|
Disopyramide |
↑↓ ↑↓ |
↑↓ |
↑↓ |
↑↑ ↑↑ |
↑↑ |
|
Flecainide |
None ↓ None↓ |
↑ |
↑ |
↑↑↑ |
0 |
|
Lidocaine |
None |
None |
0 |
0 |
0 |
|
Mexiletine |
None |
None |
0 |
0 |
0 |
|
Procainamide |
↓ ↓ |
↑↓ ↑↓ |
↑↓ |
↑↑ |
↑↑ |
|
Propafenone |
0,↓ 0,↓ |
↑ |
↑ |
↑↑↑ |
0 |
|
Quinidine |
↑↓ ↑↓ |
↑↓ |
↑↓ |
↑↑ |
↑↑ |
|
Sotalol |
↓↓ ↓↓ |
↑↑ ↑↑ |
↑↑ |
0 |
↑↑↑ ↑↑↑ |
|
SA: Sinoatrial; AV: Atrioventricular. |
|||||
References
- Singh S, Kerndt C, et al. Mexiletine. Accessed from https://www.ncbi.nlm.nih.gov/books/NBK519045/ as accessed on Februry 20 2020.
- Monk JP, Brogden RN. Mexiletine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in the treatment of arrhythmias. Drugs.1990 Sep;40(3):374-411.
- Manolis AS, Deering TF, et al. Mexiletine: pharmacology and therapeutic use. Clin Cardiol. 1990 May;13(5):349-59.
- Schrader BJ, Bauman JL. Mexiletine: A new type I antiarrhythmic agent. Drug Intel Clin Pharm. 1986;20(4):255-60.
- Bos JM, Crotti L, et al. Mexiletine Shortens the QT Interval in Patients with Potassium Channel-Mediated Type 2 Long QT Syndrome. Circ Arrhythm Electrophysiol. 2019;12(5): e007280. doi:10.1161/CIRCEP.118.007280.
- Madadi S, Nemati M, et al. Clinical results of combined amiodarone and mexiletine therapy in refractory ventricular tachycardias. Res Cardiovasc Med 2019; 8:11-3.
- Campbell RWF, Dolder MA, et al. Comparison of procainamide and mexiletine in prevention of ventricular arrhythmias after acute myocardial infarction. Lancet. 1975;1(7919):1257-1260.
- Al-Khatib SM, Stevenson WG, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018 Oct 2;72(14): e91-e220.
- Pedersen CT, Kay GN, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm. 2014 Oct;11(10): e166-96. doi: 10.1016/j. hrthm.2014.07.024. Epub 2014 Aug 30.
- Prescribing information of MEXOHAR as accessed on March 16, 2020.
- Kreeger RW, Hammill SC.et al. New antiarrhythmic drugs: Tocainide, mexiletine, flecainide, encainide, and amiodarone. Mayo Clin Proc. 1987 Nov;62(11):1033-50.
- Central Drugs Standard Control Organisation. Accessed from https://cdscoonline.gov.in/CDSCO/Drugs. as accessed on March 16, 2020.
- Waleffe A, Mary-Rabine L, et al. Combined mexiletine and amiodarone treatment of refractory recurrent ventricular tachycardia. Am Heart J. 1980;100 (6 Pt 1):788–793.
- Ravid S, Lampert S, et al. Effect of the combination of low-dose mexiletine and metoprolol on ventricular arrhythmia. Clin Cardiol. 1991 Dec;14(12):9515.
- Sobiech M., et al. Efficacy and tolerability of mexiletine treatment in patients with recurrent ventricular tachyarrhythmias and implantable cardioverter-defibrillator shocks. Kardiol Pol. 2017;75(10): 1027-32.
- Gao D, Van Herendael H, et al. Mexiletine as an adjunctive therapy to amiodarone reduces the frequency of ventricular tachyarrhythmia events in patients with an implantable defibrillator. J. Cardiovasc Pharmacol. 2013;62(2):199-204.
- Mazzanti A, Maragna R, et al. Gene-specific therapy with mexiletine reduces arrhythmic events in patients with long QT syndrome type 3. J Am Coll Cardiol. 2016; 67(9):1053-1058.
- Vecchio N. Potential Drug Interactions in Critically Ill Patients: Sacubitril/Valsartan and Mexiletine. Cardiology. 2019;142(2):81-82.
- Kobayashi A, Yamazaki N, et al. Efficacy of combination therapy with mexiletine and a low dose of propranolol for premature ventricular arrhythmias. Jpn Circ J. 1990 Dec;54(12):1486-96.
- Stein J, Podrid P, et al. Effects of oral mexiletine on left and right ventricular function. Am J Cardiol. 1984 Sep 1;54(6):575-8.
- Mexiletine hydrochloride. Accessed from https://www.rxlist.com/mexitil-drug.htm#indications. as accessed on February 13, 2020.
- Hume JR, Grant AO. Basic and Clinical Pharmacology, 13th Ed. Agent used in cardiac arrhythmias. New York: McGraw-Hill Education, 2015; Chapter 14. https://doctorlib.info/pharmacology/basic-clinical-pharmacology-13/14.html as accessed on February 20, 2020.
- Prescribing information of TACHYRA as accessed on March 12, 2020.