H1N1: A Swine Flu Pandemic
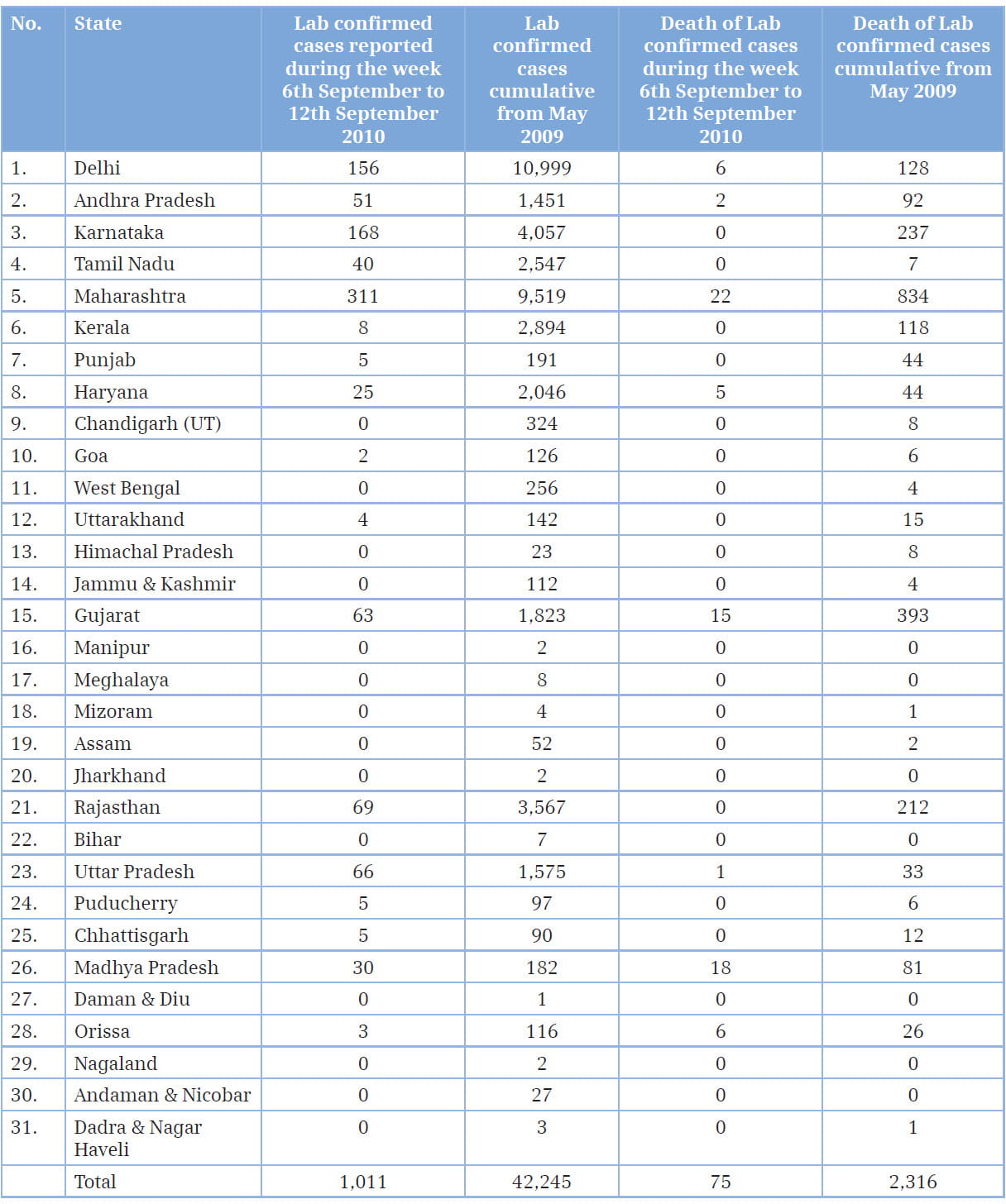
Flu is a serious contagious disease that can lead to hospitalization and even death. From 2009 - 2010, a new and very different flu virus (called 2009 H1N1) spread worldwide, causing the first flu pandemic in more than 40 years. Flu is unpredictable, but the Centers for Disease Control and Prevention (CDC), USA, expect the 2009 H1N1 virus to spread in upcoming seasons along with other seasonal flu viruses. The influenza virus, known to be circulating as a pathogen in the human population since at least the 16th century, is notable for its unique ability to cause recurrent epidemics and global pandemics. Genetic re-assortments in the influenza virus cause fast and unpredictable antigenic changes in important immune targets, leading to recurrent epidemics of febrile respiratory disease every 1-3 years consistently, necessitated the development of new vaccines. Each century has seen some pandemics rapidly progressing to all parts of the world due to the emergence of a novel virus to which the overall population holds no immunity.1 For the global health regulator, the World Health Organization (WHO), at present, the H1N1 swine flu pandemic is over. The world is no longer in an influenza pandemic alert and is moving into the post-pandemic period, as stated by the WHO Director General, Dr. Margaret Chan, in August 2010. According to the WHO, the new H1N1 virus has largely run its course. But for India, the reality seems to be different. Some say the pandemic is actually peaking in the country now. Take, for example, the number of new cases in August; between August 2 and 8, 2010, the number of lab-confirmed cases of H1N1 infection stood at 942. According to the Ministry of Health and Family Welfare, India, 167,846 persons were tested for H1N1 till August 29, with 39,977 positive and 2,113 dead. The worst affected states included Maharashtra, Karnataka, Andhra Pradesh and the capital city of Delhi.2,3 This article review the virology, epidemiology, pathogenesis and clinical data of 2009 H1N1 infections and summarizes key concerns regarding control, prevention and antiviral resistance for clinicians worldwide.
Swine influenza (swine flu) is actually a respiratory disease of pigs caused by the type A influenza virus, which was first isolated from a pig in 1930. The swine flu viruses cause high levels of illness and low death rates in pigs. These viruses usually circulate among swine throughout the year, but most outbreaks occur during the late fall and winter months, similar to outbreaks in humans. Like all influenza viruses, the swine flu viruses change constantly. Pigs can be infected by avian influenza viruses, human influenza viruses as well as swine influenza viruses and, hence, pigs are known to be a mixing vessel. When influenza viruses from different species infect pigs, the viruses can re-assort (i.e., swap genes) and new viruses (a mix of swine, human and/or avian influenza viruses) can emerge, leading to the development of new novel strains against which human beings do not have any immunity. There are four main influenza type A virus subtypes that have been isolated in pigs: H1N1, H1N2, H3N1 and H3N2. However, most of the recently isolated influenza viruses from pigs have been H1N1 viruses. Swine flu viruses do not normally infect humans. However, sporadic human infections with swine flu have occurred. Most commonly, these cases occur in persons having direct exposure to pigs. But, since 2009, there were several cases of human swine influenza A (H1N1) virus infection reported in several countries. This was a novel influenza A virus that had not been identified in people before and human-to-human transmission of the virus appeared to be ongoing; thus, this represented a real pandemic threat. As this pandemic speeded, the WHO upgraded the phasing of influenza from Phase-3 to Phase-6. Currently, according to the WHO, the new H1N1 virus has largely run its course and the world is no longer on an influenza pandemic alert and is moving into the post-pandemic period. The post-pandemic period does not mean that the H1N1 virus has gone away. The WHO says that based on experience with past pandemics, the H1N1 virus could take on the behavior of a seasonal influenza virus and continue to circulate for some years to come.

Most illnesses caused by the 2009 H1N1 virus have been acute and self-limited, with the highest attack rates reported among children and young adults.10,11,12 The relative sparing of adults older than 60 years of age was presumably due to the exposure of persons in this age group to antigenically-related influenza viruses earlier in life, resulting in the development of cross-protective antibodies. In contrast to seasonal influenza, most of the serious illnesses caused by the pandemic virus have occurred among children and non-elderly adults,and approximately 90% of deaths have occurred in those below 65 years of age. Rates of hospitalization and death have varied widely according to country.13 Hospitalization rates have been highest for children below the age of 5 years,13 especially those below the age of 1 year, and lowest for persons who were 65 years of age or older. 14 In the USA, 32-45% of patients who were hospitalized with pandemic influenza were below the age of 18 years.14,15 Approximately 9-31% of hospitalized patients had been admitted to an intensive care unit (ICU), where 14-46% of patients had died.14,16 The overall case fatality rate among hospitalized patients appears to have been highest among those 50 years of age or older and the lowest among children.9,12,14,16 The mechanisms of person-to-person transmission of the 2009 H1N1 virus appear to be similar to those of seasonal influenza, but the relative contributions of small-particle aerosols, large droplets and fomites are uncertain. Rates of secondary outbreaks of illness vary according to the setting and the exposed population, but estimates range from 4% to 28%. Many outbreaks have occurred in schools, day-care facilities, camps and hospitals. 4 Many would say the virus has now settled down to replace the seasonal influenza strain. But, influenza activity is currently most intense in the temperate areas of the Southern Hemisphere and southern Asia; H1N1 still continues to infect Indians in large numbers. The reality seems to be very different in Asian countries. The pandemic is actually peaking in a country like India. In the post-pandemic period, localized outbreaks of different magnitude may show significant levels of H1N1 transmission. India is still experiencing a countrywide outbreak of H1N1 (2009), with active transmission and a substantial number of fatal cases in several states across the country.5
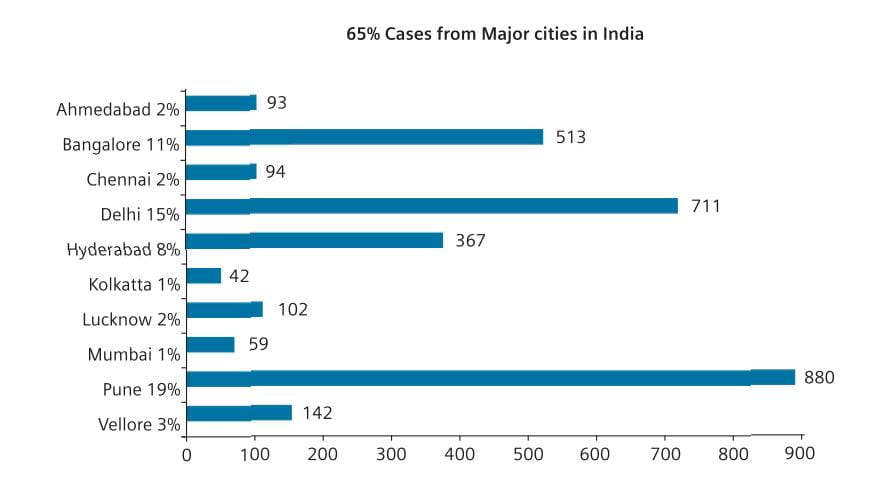
Currently, according to the Ministry of Health and Family Welfare, India, 167,846 persons were tested for H1N1 till August 29, 2010, with 39,977 positive and 2,113 were dead. The worst affected states included Maharashtra, Karnataka, Andhra Pradesh and capital city of Delhi. Up to 29th August 2010, the number of H1N1 cases detected from major cities of India amounted to 65%.3
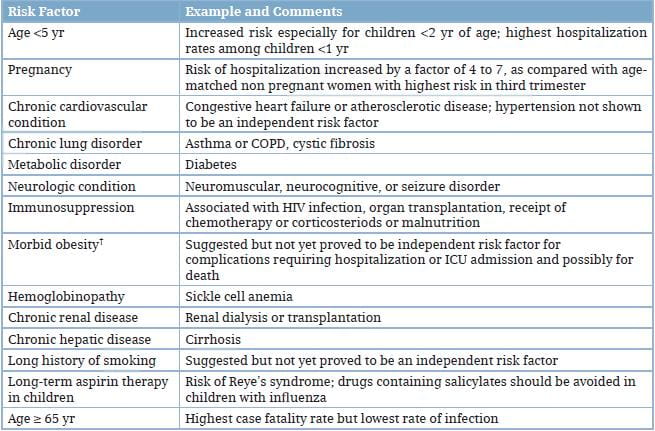
Underlying conditions that are associated with complications from seasonal influenza also are the risk factors for complications from the 2009 H1N1 virus infection (Table 1).
Studies of hemagglutinin-receptor binding indicate that the 2009 H1N1 virus is well adapted to mammalian hosts and binds to both alpha 2,6-linked cellular receptors (as do seasonal influenza viruses) and alpha 2,3-linked receptors,17 which are present in the conjunctivae, distal airways, and alveolar pneumocytes. The 2009 H1N1 virus shows increased ex vivo replication in the human bronchial epithelium at 33°C, as compared with a seasonal influenza virus,18 and is also characterized by increased replication and pathological changes in the lungs of non-human primates and increased replication in ex vivo human lung tissues.19 In uncomplicated illness, nasopharyngeal viral RNA loads peak on the day of the onset of symptoms and decline gradually afterwards. 4 However, viral replication may be more prolonged than in seasonal influenza; on day 8 of uncomplicated illness in adults and teenagers, nasopharyngeal swabs have yielded viral RNA in 74% of patients and infectious virus in 13% of patients.4 Infectious virus has been recovered from children up to 6 days after the resolution of fever. Nasopharyngeal viral loads are increased in patients with severe pneumonia and decline slowly in critically ill patients.23 Among intubated patients, viral RNA has been detected at higher levels and for longer periods in the lower respiratory tract than in the upper respiratory tract.21 Viral RNA may be detected in secretions from the lower respiratory tract up to 28 days after the onset of severe pneumonia,4 and longer in patients with immunosuppression. Viral RNA and (infrequently) infectious virus have been detected in the stool of patients, and viral RNA has been detected infrequently in the blood or urine of patients.21,23
The patterns of innate and adaptive immune responses in patients with the 2009 H1N1 virus infection are incompletely characterized. Seasonal and pandemic 2009 H1N1 viruses induce similar pro-inflammatory mediator responses in human cells in vitro,18 but do not activate effective innate antiviral responses in the human dendritic cells and macrophages.20 Increased plasma levels of interleukin-15, interleukin-12p70, interleukin-8 and, especially, interleukin-6 may be markers of critical illness.21,22 High systemic levels of interferon-ϒ and mediators involved in the development of type 1 and type 17 helper T-cell responses have been reported in hospitalized patients.22As compared with patients with less severe illness, patients who died or who had acute respiratory distress syndrome (ARDS) had increased plasma levels of interleukin-6, interleukin-10 and interleukin-15 throughout the illness,23 and of the granulocyte colony-stimulating factor, interleukin-1alpha, interleukin-8, interferon-inducible protein 10, and tumor necrosis factor alpha during the late phase of illness. Levels of serum hemagglutination-inhibition and neutralizing antibodies rise promptly after infection in immunocompetent persons,24but symptomatic re-infections have been reported.25
In fatal cases of H1N1 virus infection, the most consistent histopathological findings are varying degrees of diffuse alveolar damage, with hyaline membranes and septal edema, tracheitis, and necrotizing bronchiolitis (Figure 1). Other early changes include pulmonary vascular congestion and, in some cases, alveolar hemorrhage. In addition to infecting cells in the upper respiratory and tracheo-bronchial epithelium and mucosal glands, the 2009 H1N1 virus targets alveolar lining cells (type I and II pneumocytes) (Figure 2). Viral antigens have been readily detectable in about two-thirds of patients who died within 10 days after the onset of illness and may be detectable for more than 10 days.4Other autopsy findings include hemophagocytosis, pulmonary thromboemboli and hemorrhage, and myocarditis.23 Bronchopneumonia with evidence of bacterial co-infection has been found in 26-38% of fatal cases.27
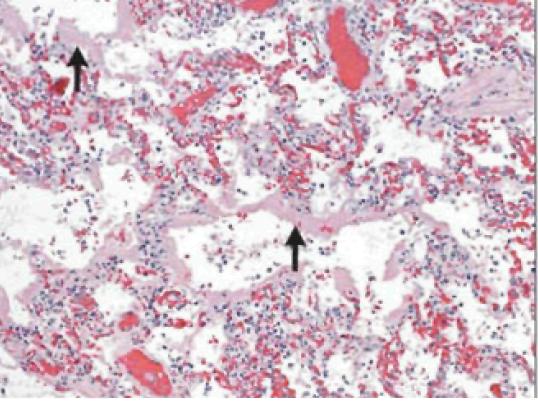
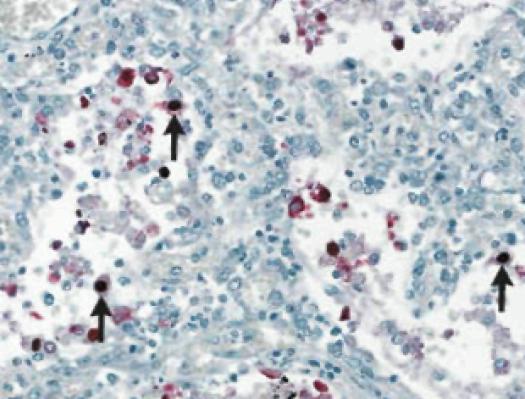
The incubation period appears to be approximately 1.5-3 days, which is similar to that of seasonal influenza. In a minority of patients, the period may extend to 7 days.
Infection with the 2009 H1N1 virus causes a broad spectrum of clinical syndromes, ranging from a febrile upper respiratory illness to fulminant viral pneumonia. Mild illness without fever has been reported in 8-32% of infected persons. Most patients presenting for care have typical influenza-like illness with fever and cough, symptoms that are sometimes accompanied by sore throat and rhinorrhea. Systemic symptoms are frequent. Gastrointestinal symptoms (including nausea, vomiting and diarrhea) occur more commonly than in seasonal influenza, especially in adults. Dyspnea, tachypnea in children, chest pain, hemoptysis or purulent sputum, prolonged or recurrent fever, altered mental status, manifestations of dehydration, and reappearance of lower respiratory tract symptoms after improvement are signs of progression to more severe disease or complications. The principal clinical syndrome leading to hospitalization and intensive care is diffuse viral pneumonitis associated with severe hypoxemia, ARDS and, sometimes, shock and renal failure. This syndrome has accounted for approximately 49-72% of ICU admissions for the 2009 H1N1 virus infection. Rapid progression is common, typically starting on days 4-5 after the onset of illness, and intubation is often necessary within 24 hours after admission. Currently available prognostic algorithms for community-acquired pneumonia, such as CURB-65 (a measure of confusion, urea nitrogen, respiratory rate and blood pressure, and an age of 65 years or older), may not apply. Radiographic findings commonly include diffuse mixed interstitial and alveolar infiltrates, although lobar and multilobar distributions occur, particularly in patients with bacterial co-infection. Chest computed tomography has shown multiple areas of ground-glass opacities, air bronchograms and alveolar consolidation, particularly in the lower lobes. Small pleural effusions occur, but an increased volume suggests volume overload or possibly empyema. Pulmonary thromboemboli have occurred in some critically ill patients with ARDS. Other important syndromes include severe, prolonged exacerbation of chronic obstructive pulmonary disease (COPD) or asthma (in about 14-15% of patients), bacterial co-infections, and decompensation of serious coexisting conditions (Table 1). Among hospitalized patients with 2009 H1N1 infection, a history of asthma has been reported in 24-50% of children and adults, and COPD in 36% of adults. Bacterial pneumonia, usually caused by Staphylococcus aureus (methicillin-resistant), Streptococcus pneumoniae, S. pyogenes and, sometimes, other bacteria, has been suspected or diagnosed in 20-24% of ICU patients and has been found in 26-38% of patients who died, often in association with a short clinical course. Death from the 2009 H1N1 virus and bacterial co-infection has occurred within 2-3 days in some cases. Sporadic cases of neurologic manifestations (confusion, seizures, unconsciousness, acute or post-infectious encephalopathy, quadriparesis and encephalitis) and myocarditis have been reported, including some fulminant cases. Laboratory findings at presentation in patients with severe disease typically include normal or low-normal leukocyte counts, with lymphocytopenia and elevations in levels of serum aminotransferases, lactate dehydrogenase, creatine kinase and creatinine. Myositis and rhabdomyolysis have occurred in severe cases. A poor prognosis is associated with increased levels of creatine kinase, creatinine and, perhaps, lactate dehydrogenase, along with the presence of thrombocytopenia and metabolic acidosis (Table 3).4
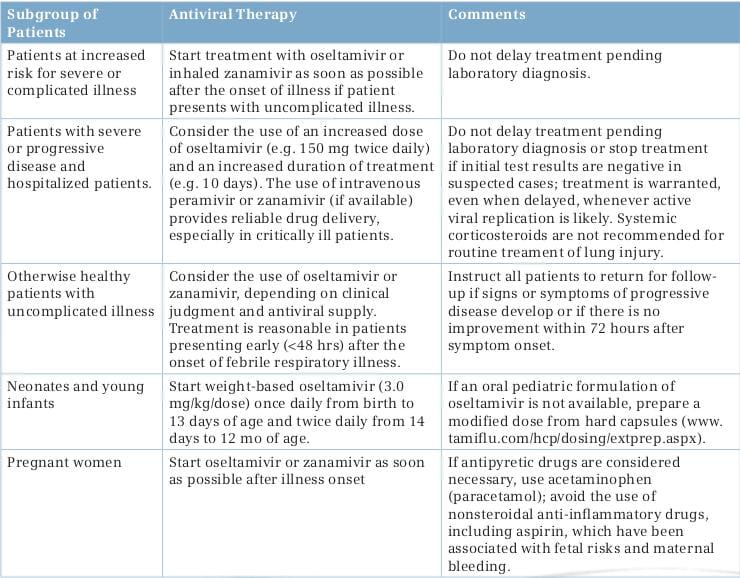
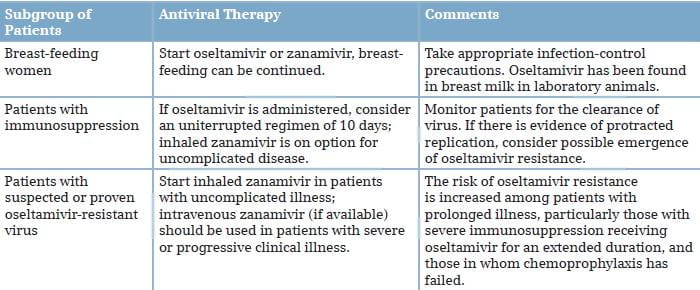
Viral RNA detection by conventional or real-time reverse-transcriptase-polymerase-chain-reaction (rRT-PCR) assay remains the best method for the initial diagnosis of the H1N1 virus infection. Nasopharyngeal aspirates or swabs taken early after the onset of symptoms are suitable samples, but endotracheal or bronchoscopic aspirates have higher yields in patients with lower respiratory tract illness. One study showed that among patients with detectable H1N1 viral RNA in bronchoscopic samples, 19% had negative upper respiratory tract samples. Negative lower respiratory tract samples have been noted in 10% or more of patients with severe H1N1 virus infection. Consequently, negative results in single respiratory specimens do not rule out the H1N1 virus infection, and repeated collection of multiple respiratory specimen types is recommended when clinical suspicion is high. Commercially available rapid influenza antigen assays have poor clinical sensitivity (11-70%) for the detection of the 2009 H1N1 virus in respiratory specimens and cannot differentiate among influenza A subtypes. Consequently, negative test results should not be used to make decisions with respect to treatment or infection control. Direct or indirect immunofluorescence tests are less sensitive than rRT-PCR. The H1N1 virus replicates in various cell types, but isolation usually takes several days. Serologic assays (microneutralization and hemagglutination inhibition) that detect increases in antibody levels in paired serum samples provide a retrospective diagnosis; single high titers in serum samples from convalescent patients may be indicative of recent infection, but routine testing of a single specimen to detect recent infection is not recommended.
Treatment should be started empirically, based on clinical judgment, as early as possible even before definitive diagnostic test results become available, i.e., treatment should not wait for laboratory confirmation of influenza. Treatment is most effective when started in the first 48 hours of illness. As noted above, however, evidence suggests that treatment may benefit patients with prolonged or severe illness even when started more than 48 hours after the onset of illness.
Definitive testing for 2009 H1N1 requires rRT-PCR or viral culture. These tests should be prioritized for persons with suspected or confirmed influenza requiring hospitalization and should be based on guidelines from the local and state health departments.
Rapid influenza diagnostic tests (RIDTs) should not be used to rule out influenza because false-negative results are common. The sensitivity of rapid tests in detecting 2009 H1N1 has ranged from 10% to 70%. Clinicians should not withhold treatment based on a negative rapid test result. Information on the use of RIDTs is available online.28
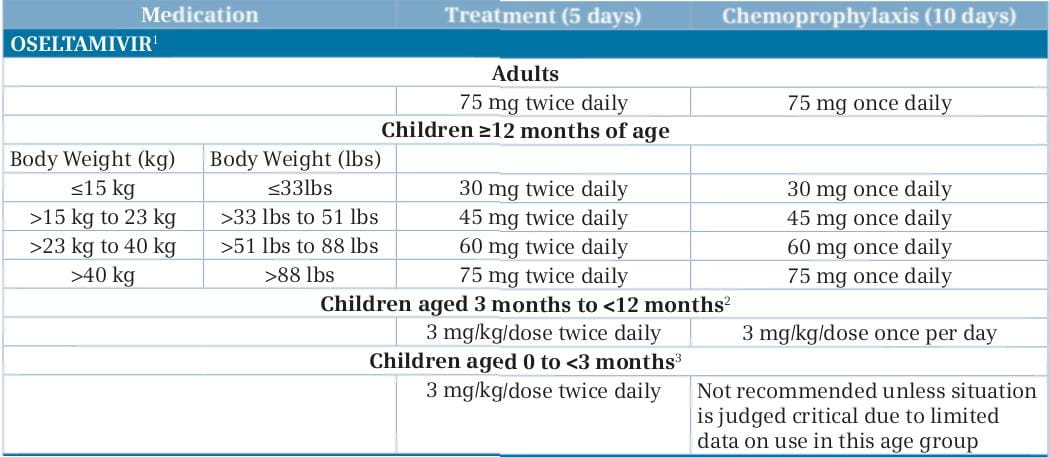
The currently circulating H1N1 virus is susceptible to the neuraminidase inhibitors,oseltamivir (Anti-flu) and zanamivir (Virenza), but is almost always resistant to amantadine and rimantadine.
4, 7 Presently, both oseltamivir and zanamivir are the choices for the treatment of H1N1 influenza and influenza-like illness in both children and adults.
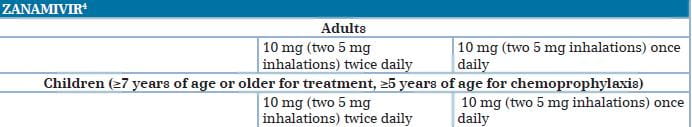
Therapy with a neuraminidase inhibitor is especially important for patients with underlying risk factors, including pregnancy, and those with severe or progressive clinical illness as shown in Table 3. Standard doses of oseltamivir or inhaled zanamivir can be used for the treatment of mild illness, unless viral resistance to oseltamivir has been documented or is suspected (e.g., because of chemoprophylaxis failure); in this case, zanamivir is preferred.
Early therapy with oseltamivir in patients with the H1N1 virus infection may reduce the duration of hospitalization and the risk of progression to severe disease requiring ICU admission, or resulting in death. In one study involving 45 patients with the H1N1 virus who had cancer or had undergone hematopoietic stem-cell transplantation, 18% had pneumonia and 37% were hospitalized; all patients received oseltamivir, and no deaths were reported.4 Presently, these neuraminidase inhibitors are the drugs of choice for the treatment of H1N1 influenza and influenza-like illness in both children and adults.
Oseltamivir: The neuraminidase inhibitor, oseltamivir (formulated as capsules or oral suspension) is FDA-approved for the treatment of uncomplicated acute influenza in patients, 1 year of age and older, who have been symptomatic for no more than 2 days. The FDA has issued an Emergency Use Authorization (EUA), authorizing treatment with oseltamivir of patients less than 1 year old with H1N1 influenza. In addition, the EUA authorizes treatment of patients symptomatic with the 2009 H1N1 influenza for more than 2 days and patients sick enough to require hospitalization.8
Zanamivir: The neuraminidase inhibitor, zanamivir, formulated for oral inhalation, is FDA-approved for the treatment of influenza in patients, 7 years of age and older, who, similar to approved uses for oseltamivir, have uncomplicated illness and have been symptomatic for no more than 2 days. As with oseltamivir, the FDA has issued an EUA authorizing treatment with zanamivir of patients with the 2009 H1N1 influenza who have been symptomatic for more than 2 days and patients sick enough to require hospitalization.8
Peramivir: A third neuraminidase inhibitor, peramivir, formulated for intravenous (I.V.) administration, is an investigational product currently being evaluated in clinical trials. As of October 2009, safety and/or efficacy data from 1,891 patients with acute uncomplicated seasonal influenza A has been submitted to the FDA. Efficacy and safety have not been evaluated in hospitalized patients. Even though the data are insufficient to allow FDA approval, the FDA issued an EUA for treatment with peramivir of hospitalized patients with the 2009 H1N1 influenza who have potentially life-threatening suspected or laboratory-confirmed infection.
Peramivir I.V. Is available through the CDC upon the request of a licensed physician. Under the EUA, treatment of adult patients with I.V. Peramivir is approved only if (1) the patient has not responded to either oral or inhaled antiviral therapy; (2) drug delivery by a route other than I.V. Is not expected to be dependable or is not feasible; or (3) the clinician judges I.V. therapy is appropriate due to other circumstances. Treatment of pediatric patients is approved if either of the first two criteria applies.8
People who are already recovering from influenza do not need antiviral medications for treatment. Options for close follow-up should be carefully considered. Clinicians who prefer not to treat empirically should discuss the signs and symptoms of worsening illness with such patients and arrange for a follow-up at least by telephone.
A His275 Tyr mutation in viral neuraminidase confers high-level resistance to oseltamivir, but not to zanamivir. The situation of oseltamivir resistance in swine flu is the present concern of medical society. The oseltamivir resistance in swine flu is expected to be possible due to etiologies. According to the WHO, oseltamivir resistance from the pandemic influenza A (H1N1) 2009 virus has been reported in totally 302 cases so far, where all but one had the H275Y substitution and is assumed to remain sensitive to zanamivir.
If generalized oseltamivir resistance occurs, there is no doubt that another authorized antiviral drug, zanamivir has to be used. However, the expectation of a rapid resistance to zanamivir can be imagined since zanamivir is a drug in the same group as oseltamivir. This is the reason for the urgent need to search for a new drug.
Both the I.V. neuraminidase inhibitors, zanamivir and peramivir, provide rapid drug delivery at high levels, but both are investigational drugs. Both drugs are available on a compassionate-use basis for treating seriously ill patients, and peramivir is authorized for emergency use in hospitalized patients in the USA, and licensed for use in Japan. In one study of adults hospitalized with seasonal influenza, the efficacy of I.V. peramivir appeared to be similar to that of oseltamivir, but peramivir is less active by a factor of at least 80 for oseltamivir-resistant viruses carrying the His275Tyr mutation than for oseltamivir-susceptible viruses. I.V. zanamivir (if available) is, currently, the preferred option for hospitalized patients with suspected or documented oseltamivir-resistant 2009 H1N1 virus infection.4, 29, 30
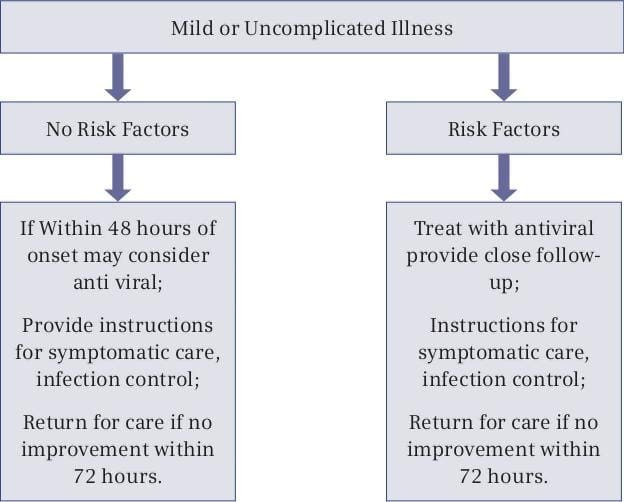
Treatment is recommended for patients with confirmed or suspected H1N1 influenza who have severe, complicated or progressive illness, or who are hospitalized. The recommended duration of treatment is 5 days. Hospitalized patients with severe infections (such as those with prolonged infection or who require ICU admission) might require longer treatment courses. Even though treatment is most effective when started in the first 48 hours of illness, limited data from observational studies of hospitalized patients suggests treatment of persons with prolonged or severe illness reduces mortality or duration of hospitalization even when treatment is started more than 48 hours after the onset of illness.
There are two types of vaccines:
- The "flu shot" - an inactivated vaccine (containing killed virus) that is given with a needle, usually in the arm. The flu shot is approved for use in people older than 6 months of age, including healthy people and people with chronic medical conditions.
- The nasal spray flu vaccine - a vaccine made with live, weakened flu viruses that do not cause the flu (sometimes called LAIV for "live attenuated influenza vaccine"), which is approved for use in healthy* people, 2 to 49 years of age, who are not pregnant.
The flu vaccine is updated every year to combat the flu viruses that research indicates are most likely to cause illness during the upcoming season. The 2010 - 2011 flu vaccine is being made in the same way as seasonal vaccines have been made for decades. It will protect against the 2009 H1N1 virus that caused so much illness last season, and two other influenza viruses (an H3N2 virus and an influenza B virus). About 2 weeks after vaccination, antibodies that provide protection against the influenza virus infection develop in the body.
Even people who got vaccinated with the 2009 H1N1 vaccine or last year's seasonal vaccine need to be vaccinated with the flu seasonal vaccine this year. This season's vaccine provides protection against other influenza strains that were not in either the seasonal or the 2009 H1N1 vaccine last season; besides, immunity from a vaccine taken last year may decline over time.31
The CDC urges people to take the following actions to protect themselves and others from influenza (the flu):
Take time to get a flu vaccine.
- The CDC recommends a yearly flu vaccine as the first and most important step in protecting against flu viruses. There are many different flu viruses; the flu vaccine protects against the three viruses that research suggests will be most common.
- The 2010 -2011 flu vaccine will protect against an influenza A H3N2 virus, an influenza B virus and the 2009 H1N1 virus that caused so much illness last season.
- Everyone who is 6 months of age and older should get vaccinated against the flu as soon as the 2010 -2011 season vaccine is available. Vaccination of high-risk persons is especially important to decrease their risk of contracting severe flu illness.
- Vaccination also is important for healthcare workers, and other people who live with or care for high-risk people so as to prevent the spread of the flu to high-risk people.
- Children younger than 6 months of age are at high risk of developing serious flu illness, but are too young to be vaccinated. People who care for them should be vaccinated instead.
- Cover your nose and mouth with a tissue when you cough or sneeze. Throw the tissue in the trash after you use it.
- Wash your hands often with soap and water. If soap and water are not available, use an alcohol-based hand rub.
- Avoid touching your eyes, nose and mouth. Germs spread this way.
- Try to avoid close contact with sick people.
- If you are sick with a flu-like illness, the CDC recommends that you stay home for at last 24 hours after your fever is gone, except to get medical care or for other necessities. (Your fever should be gone without the use of a fever-reducing medicine.)
- While sick, limit contact with others as much as possible to keep from infecting them.
- It is very important that antiviral drugs be used early (within the first 2 days of symptoms) to treat people who are very sick (such as those who are hospitalized) or people who are sick with flu symptoms and who are at increased risk of severe flu illness, such as pregnant women, young children, people aged 65 years and older, and people with certain chronic health conditions.
Visit the CDC website below to find out what to do if you get sick with the flu and how to care for someone at home who is sick with the flu.32
http://www.cdc.gov/flu/protect/preventing.htm
An outbreak of human infection with a novel influenza A (H1N1) virus of swine origin is spreading through sustained human to human transmission in multiple countries. According to the Ministry of Health and Family Welfare, India, 2,113 deaths were reported due to H1N1 till August 29, with 39,977 positive cases. According to the WHO experience with past pandemics, the H1N1 virus could take on the behavior of a seasonal influenza virus and continue to circulate for some years to come. Hence, a complete understanding of the virology, epidemiology, and pathogenesis of H1N1 infections is necessary, because prevention and control measures for H1N1 virus are based on our understanding of seasonal human influenza and consideration of potential modes of transmission.
Currently, two classes of antiviral medication are available for the treatment of seasonal human influenza: neuraminidase inhibitors (oseltamivir and zanamivir) and adamantanes (rimantadine and amantadine). During the 2008-2009 influenza season, almost all circulating human influenza A (H1N1) viruses in the United States were resistant to oseltamivir. However, genetic and phenotypic analyses indicate that H1N1 virus was susceptible to oseltamivir and zanamivir but resistant to the adamantanes; the CDC has recommended that given the severity of illness observed among some patients with H1N1 virus, therapy with neuraminidase inhibitors should be prioritized for hospitalized patients with suspected or confirmed H1N1 infection and for patients who are at high risk for complications from seasonal influenza. The CDC also recommends a yearly flu vaccine as the first and most important step in protecting against flu viruses. All recommendations as and when updated are posted on the CDC's website at www.cdc.gov/h1n1flu/recommendations.htm
In conclusion, enhanced surveillance for H1N1 infection is implemented globally; additional cases are expected to be identified. The current trends for H1N1 infections in India as described in this article may provide guidance for clinicians with respect to presenting symptoms and outcomes of infection with this novel virus, thus helping us prevent the future epidemic.
1. CD Alert Monthly Newsletter of the National Institute of Communicable Diseases, Directorate General of Health Services,Government of India, March -April 2009; Vol.12 : No. 8 Special Issue: Human Swine Influenza: A pandemic threat.
2. In 7 days, H1N1 claims 83 lives - The Times of India http://timesofindia.indiatimes.com/india/In-7-days-H1N1-claims-83 lives/articleshow/6316774.cms#ixzz0zlp70NdT accessed in September 2010.
3. http://mohfw-h1n1.nic.in/index.html accessed in September 2010.
4. N Engl J Med 2010; 362:1708-19.
5.http://www.who.int/csr/disease/influenza/2010_09_10_GIP_surveillance/en/index.html accessed in September 2010.
6. http://pib.nic.in/release/release.asp?relid=65723 accessed in September 2010.
7. N Engl J Med 2009; 360:2605-15.
8. http://www.cdc.gov/H1N1flu/recommendations.htm
9. Echevarria-Zuno S, Mejia-Arangur1. e JM, Mar-Obeso AJ et al. Infection and death from influenza A H1N1 virus in Mexico: A retrospective analysis. Lancet 2009; 374:2072-9.
10. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360:2605-15. [Erratum, N Engl J Med 2009; 361:102]
11. Reed C, Angulo FJ, Swerdlow DL et al. Estimates of the prevalence of pandemic (H1N1) 2009, United States, April-July 2009. Emerg Infect Dis 2009; 15:2004-7.
12. Donaldson LJ, Rutter PD, Ellis BM et al. Mortality from pandemic A/H1N1 2009 influenza in England: Public health surveillance study. BMJ 2009; 339:b5213.
13. Transmission dynamics and impact of pandemic influenza A (H1N1) 2009 virus. Wkly Epidemiol Rec 2009; 84:481-4.
14. Louie JK, Acosta M, Winter K et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A (H1N1) infection in California. JAMA 2009; 302:1896-902.
15. Jain S, Kamimoto L, Bramley AM et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med 2009; 361:1935-44.
16. Kumar A, Zarychanski R, Pinto R et al. Critically ill patients with 2009 influenza A (H1N1) infection in Canada, JAMA 2009; 302:1872-9.
17. Childs RA, Palma AS, Wharton S et al. Receptor-binding specificity of pandemic influenza A (H1N1) 2009 virus determined by carbohydrate microarray. Nat Biotechnol 2009; 27:797-9.
18. Chan MC, Chan RW, Yu WC et al. Tropism and innate host responses of the 2009 pandemic H1N1 influenza virus in ex vivo and in vitro cultures of human conjunctiva and respiratory tract. Am J Pathol 2010; 176:1828-40.
19. Itoh Y, Shinya K, Kiso M et al. in vitro and in vivo characterization of new swine origin H1N1 influenza viruses. Nature 2009; 460:1021-5.
20. Osterlund P, Pirhonen J, Ikonen N et al. Pandemic H1N1 2009 influenza A virus induces weak cytokine responses in human macrophages and dendritic cells and is highly sensitive to the antiviral actions of interferons. J Virol 2010; 84:1414-22.
21. Lee N. Pathogenesis of pandemic H1N1 in humans. Presented at the XII International Symposium on Respiratory Viral Infections, Taipei, Taiwan, March 11-14, 2010. Abstract.
22. Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care 2009; 13:R201.
23. To KK, Hung IF, Li IW et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis 2010; 50:850-9.
24. Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: A cross-sectional serological study. Lancet 2010; 375:1100-8.
25. Perez CM, Ferres M, Labarca JA. Pandemic (H1N1) 2009 re-infection, Chile. Emerg Infect Dis 2010; 16:156-7.
26. Shieh WJ, Blau DM, Denison AM et al. Pandemic influenza A (H1N1): Pathology and pathogenesis of 100 fatal cases in the U.S. Am J Pathol (in press).
27. Mauad T, Hajjar LA, Callegari GD et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med 2010; 181:72-9.
28. http://www.cdc.gov/h1n1flu/recommendations.htm accessed in September 2010.
29. North American Journal of Medical Sciences 2009, 1(3).
30. http://www.who.int/csr/disease/swineflu/oseltamivirresistant20100806.pdf accessed in September 2010.
31. http://www.cdc.gov/flu/protect/vaccine/fluvax_whatsnew.htm accessed in September 2010.
32. http://www.cdc.gov/flu/protect/preventing.htm accessed in September 2010.