Gutron (Midodrine hydrochloride) Monograph
Overview of Hypertension
Introduction
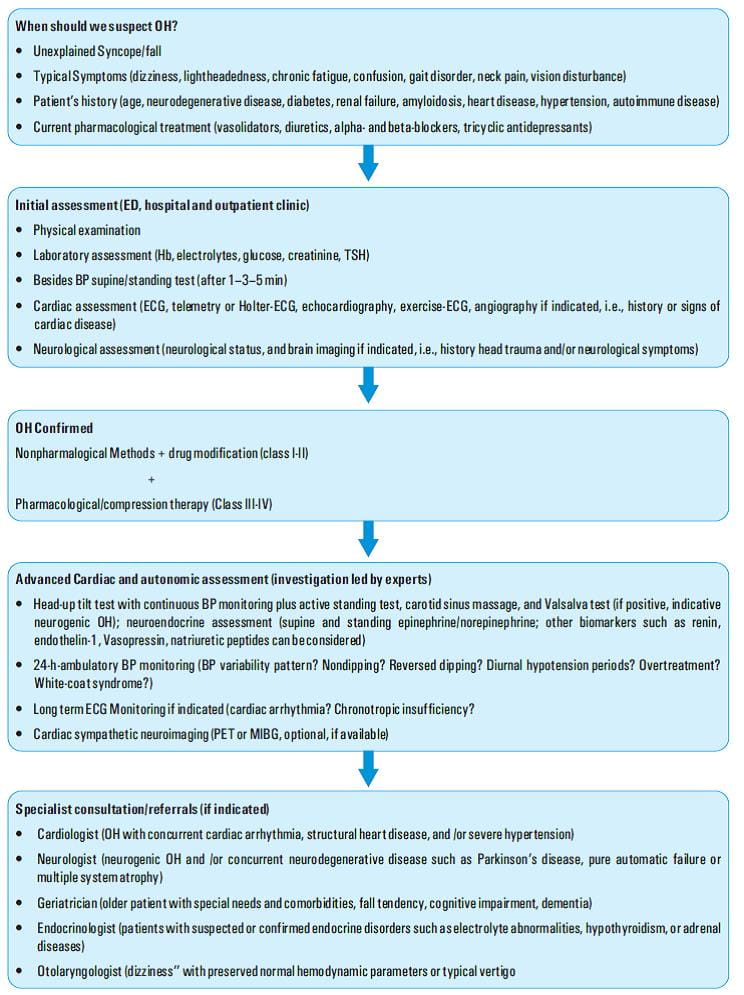
Orthostatic hypotension (OH) is commonly encountered among elderly patients. The incidence of OH and syncope increases with age, being more common in elderly residents of health care facilities (54%–68%) than in those in the community (6%).1-6 In a meta-analysis of diabetic patients, the pooled prevalence of OH was found to be 24%.7
OH is usually asymptomatic, but most patients with asymptomatic OH have subtle symptoms in conditions of increased orthostatic stress (for e.g., post meal, during raised ambient temperature or after exertion). Light-headedness is common, but many patients have subtle symptoms, such as tiredness or difficulty in concentration and thinking.
Diverse factors have been implicated in the development of OH in elderly including medications, fluid or blood loss, adrenal insufficiency, altered blood pressure regulatory mechanisms, autonomic dysfunction and underlying disease process. Neurological diseases such as diabetic neuropathy, Parkinson's disease, multiple system atrophy and the autonomic neuropathies exacerbate the risk of OH. The presence of multiple co-morbid conditions and the non-specificity of signs and symptoms pose a challenge for making the diagnosis of OH.8
Orthostatic hypotension is associated with significant morbidity and mortality. The management of orthostatic hypotension poses a challenge. Non-pharmacological treatment options are the first approach. When this fails, drug treatment is initiated. The different drugs used in the management of OH include midodrine, pyridostigmine, droxidopa and fludrocortisone. Midodrine is an effective treatment for symptomatic OH and is well-tolerated9. Monotherapy or combination treatment with midodrine and pyridostigmine is effective and safe in patients with OH for up to 3 months. Midodrine is superior to pyridostigmine for improving OH-related symptoms.10
Physiological Mechanisms Involved in the Maintenance of Normal Orthostatic Response
Several physiologic mechanisms have been implicated in the development of orthostatic hypotension (Table 1).11The two chief systems involved in the maintenance of a normal hemodynamic response to changes in posture are the cardiovascular and autonomic nervous systems.11A change in posture causes pooling of about 500 to 1,000 mL of blood in the lower extremities and splanchnic circulation. This begins the cascade of increasing the sympathetic outflow, peripheral vascular resistance, venous return, and cardiac output and effectively counteracts the decrease in blood pressure. These compensatory mechanisms cause a decrease in systolic blood pressure (SBP) 5 to 10 mmHg, an increase in diastolic blood pressure (DBP) 5 to 10 mmHg, and an increase in pulse rate (10 to 25 beats per minute).12
|
|
|
|
|
|
|
Definition of Orthostatic Hypertension
OH is defined as a sustained decrease in systolic blood pressure of 20 mmHg or a decrease in diastolic blood pressure of 10 mmHg within three minutes of standing compared with blood pressure from the sitting or supine position. If the sustained blood pressure (BP) drop occurs after three minutes of upright posture, it is called delayed OH.13
On the basis of temporal changes in orthostatic BP, 3 different clinical variants of OH have been proposed: classic, delayed and initial OH.14
Classic OH
Classic OH is defined as a sustained reduction in SBP of at least 20 mmHg and/or DBP of at least 10 mmHg, within 30 to 180 s of active standing or during a head-up tilt test of at least 60°.14A reduction in SBP of at least 30 mmHg in patients with supine hypertension, may be a more appropriate criterion for OH since the magnitude of the fall of BP is observed to be proportional to baseline values.
Classic OH is closely related to neurogenic hypotension but it features symptoms of generalized autonomic failure which are rarely observed and after extensive neurological evaluation, no identifiable etiology can be identified in almost one-third of patients.14 In patients where the structural autonomic disease cannot be confirmed, the term idiopathic OH has been adopted. Classic OH represents a key and typical manifestation of sympathetic neurocirculatory failure leading to impaired chronotropic and vascular responses during early orthostasis.14
Delayed OH
Delayed OH is caused by a gradual impairment of adaptive mechanisms during orthostasis, leading to a slow progressive decrease in arterial pressure (BP decrease 20/10 mmHg or 30/15 mmHg in patients with hypertension) between 3 and 45 min.
It is associated with milder abnormalities of sympathetic adrenergic function. Delayed OH may be a less severe or an early form of autonomic failure, occurring due to age-related impairment of compensatory reflexes. Typically, the absence of bradycardia or pauses helps to distinguish delayed OH from reflex syncope but mixed forms are often encountered in clinical practice.14
Initial OH
Initial OH is defined as a transient BP decrease (SBP decrease >40 mmHg and/or DBP decrease >20 mm Hg) within 30s of standing. Initial OH may represent an unrecognized cause of syncope and is attributed to an exaggerated and immediate transient decrease in arterial BP upon standing. It is exclusively seen with active standing, and the drop in BP during passive tilting is significantly smaller, or, may even be absent in many cases.14
The mechanism of initial OH postulated includes a short-lasting mismatch between a sudden decrease in venous return and neurally mediated compensatory vasoconstriction. The diagnosis of initial OH poses a distinct challenge and can only be confirmed by an active standing test with continuous BP monitoring.14
Prevalence of OH
The incidence of OH and syncope increases with ag, being more common in elderly residents of health care facilities (54%-68%) than in those in the community (6%).1 in a meta-analysis of diabetic patients, the pooled prevalence of OH was found to be 24%.7 The estimated prevalence of OH in different autonomic disorders is listed in Table 2.15
|
Condition |
Prevalence rate (%) |
|
Elderly |
10–30 |
|
Diabetes type I |
8.4 |
|
Diabetes type II |
7.4 |
|
Parkinson’s disease |
37–58 |
|
Dementia with Lewy bodies |
30–50 |
In India, OH is a common clinical finding in older persons, with a prevalence of 6% to 30%.16 In clinical trials in India, 13.5% patients with cardiac autonomic neuropathy were observed to have OH.17
The variable prevalence of OH globally may be attributed to the fact that OH is seldom sought in clinical practice, regardless of the recommendations of guidelines to screen for postural variations of blood pressure especially in older people. The presence of multiple co-morbid conditions and the non-specificity of signs and symptoms pose a challenge to make the diagnosis of OH. It is necessary to promote its detection by adhering to the recommendations for the blood pressure measurement in the clinical setting.18
Pathogenesis of OH
OH develops in patients in whom the compensatory mechanisms fail to resist the approximately 500 mL reduction of blood entering the heart as a person stands up from a supine position. With the reduction in the cardiac output, baroreceptors located in the heart, aorta and carotid artery are stimulated to increase the heart rate and cause peripheral vasoconstriction to maintain the blood pressure.
OH occurs due to several reasons such as inadequate intravascular volume, autonomic nervous system dysfunction, decreased venous return or inability to increase cardiac output in response to postural changes. Decreased cerebral perfusion causes the neurologic symptoms of OH.12
Age and disease related changes are implicated in the changes in physiology and the consequent increase the likelihood of hypotension in the face of cardiovascular stressors.2,4,5
- Increased arterial compliance and venous system tortuosity.
- Cardiac hypertrophy associated with aging or hypertension impairs diastolic filling.
- Renal sodium conservation declines and renin angiotensin and aldosterone levels are also lower with aging.
- With standing there is a blunting of the related hormones, as well as arginine vasopressin.
- The change in heart rate after hypotensive maneuvers and the maximum heart rate during exercise or isoproterenol infusion are decreased with age and in patients who may be receiving adrenergic antagonists.
- Diuretic-induced dehydration or drug-induced vasodilation causes greater BP reduction in the elderly as compared with younger patients.
- BP decreases with meals in older patients but not in younger patients.
- Cerebral blood flow reduces with increasing age.
Acute and Chronic Causes of OH
OH can be classified as either primary or secondary and can be further subcategorised into acute and chronic types (Table 3 and Table 4).14
Secondly, based on the pathophysiology, OH may be further sub categorised into 2 categories related to structural (neurogenic) or functional (non-neurogenic) causes of autonomic nervous system failure. Neurogenic OH is the chief manifestation of chronic autonomic failure in primary neurodegenerative disorders (pure autonomic failure, multiple system atrophy or Parkinson disease), but it has also been observed to occur secondary to neurological disturbances seen with diabetes, amyloidosis or advanced renal failure.14
|
Causes |
|
|
Medications |
Tricyclic antidepressant a1-blockers, antiparkinsonian, antihypertensives, angiotensin converting enzyme inhibitors, nitrates, calcium channel blockers, beta-blockers adrenoceptor blocker, dopamine receptor agonists, sympatholytic, diuretics |
|
Intravascular volume depletion |
Dehydration, vomiting, diarrhea, hemorrhage |
|
Cardiovascular |
Myocardial infarction, congestive heart failure |
|
Endocrine |
Adrenal insufficiency, hypoaldosteronism |
|
Disorder |
Example |
|
|
Age-related physiologic changes |
|
|
|
Autonomic neuropathy |
Primary disorders
|
|
Risk Factors of OH
- Advancing age- is associated with a number of autonomic nervous system neurodegenerative disorders.7
- Diabetes mellitus
- Cardiac autonomic neuropathy
- Medications can increase the risk of triggering OH. Drugs used in the management of hypertension, myocardial ischemia, depression, psychosis and schizophrenia, Alzheimer and Parkinson disease as well as a vaccine approved for the prevention of cervical cancer.7
Symptoms of OH
OH is usually asymptomatic, but most patients with asymptomatic OH have subtle symptoms in conditions of increased orthostatic stress (post meal, during raised ambient temperature or after exertion).19
Light-headedness is common, but many patients have subtle symptoms, such as tiredness or difficulty in concentrating and thinking. This symptom is more commonly observed in patients over 70 years of age and it significantly impairs the quality of life.19
Autonomic hyperactivity causes symptoms such as palpitations, tremulousness, anxiety and nausea and are observed to occur in patients with OH who have only partial autonomic failure. These symptoms are commonly observed in younger patients.19
The symptoms are often observed to be worse in the early morning, post meals, during a rise in core temperature, during prolonged standing and with activity. The early morning severity of OH may be related to nocturnal diuresis in many patients. Postprandial worsening of OH is observed to occur commonly within 30 min and lasts for about 1 h. A fact to consider is that paradoxically, such worsening might not be observed in advanced autonomic failure, when the splanchnic mesenteric bed cannot vasodilate postprandially.19
Effect of vasodilation: Patients often report more symptoms after a hot bath or on a hot day. In fact any stress which results in vasodilation of skin vessels will worsen symptoms. Patients who get up in the middle of the night out of a warm bed are vasodilated and they will have worse OH than if they get up from a colder environment. Symptoms might be worse after ingestion of alcohol because of vasodilation.19
Effect of physical activity: If physical activity is sufficient to cause muscle vasodilation, OH will be worse due to cerebral hypoperfusion. Syncope is seen patients with severe and sustained OH. But, syncope episodes may reduce after diagnosis, since patients learn to recognise the symptoms of OH and they often take corrective measures.17
Grading of Symptoms
With the advent of therapeutic advances, researchers proposed a quantitative approach to classify orthostatic intolerance. A formal grading scale (Table 5)19has been developed based on parameters such as the frequency and severity of symptoms, standing time before onset of symptoms, influence on activities of daily living and BP.19
A patient with grade I OH might not need drugs, whereas those patients with grades III or IV require aggressive therapy. Monitoring of the course and severity of OH or the response to therapy and the individual components of this grading scale can be graded numerically using a self-report orthostatic grading score (Table 6).
This validated scoring scheme has been developed based on the autonomic symptom profile and it correlates well with the severity and distribution of autonomic failure. The symptoms of orthostatic intolerance are scored based on the frequency, severity, response to orthostatic stressors, interference with activities of daily living, and standing time, and this gives a score ranging from 0 (no symptoms) to 20 (maximum symptoms or dysfunction) (Table 6). This scale helps to monitor the status of OH and response to therapy.19
|
Symptom grade of orthostatic intolerance |
|
Grade I Orthostatic symptoms are infrequent, inconstant, or only under conditions of increased orthostatic stress Standing time typically ≥15 min Unrestricted activities of daily living Blood pressure indices might or might not be abnormal |
|
Grade II Orthostatic symptoms develop at least once a week. Orthostatic symptoms commonly develop with orthostatic stress Standing time ≥5 min on most occasions Some limitation in activities of daily living Some change in cardiovascular indices. These might be OH, reduction in pulse pressure, ≥50% or excessive oscillations in blood pressure |
|
Grade III Orthostatic symptoms develop on most occasions, and are regularly unmasked by orthostatic stressors Standing time 1 min on most occasions Marked limitation in activities of daily living OH ≥50%of the time, recorded on different days |
|
Grade IV Orthostatic symptoms consistently present Standing time <1 min on most occasions Serious incapacitation; bed-bound or wheelchair-bound because of orthostatic intolerance. Syncope or presyncope is common if the patient attempts to stand OH is consistently present |
|
The patient is instructed to select appropriate answer |
|
Frequency of orthostatic symptoms 0 I never or rarely experience orthostatic symptoms when I stand up 1 I sometimes experience orthostatic symptoms when I stand up 2 I often experience orthostatic symptoms when I stand up 3 I usually experience orthostatic symptoms when I stand up 4 I always experience orthostatic symptoms when I stand up |
|
Severity of orthostatic symptoms 0 I do not experience orthostatic symptoms when I stand up 1 I experience mild orthostatic symptoms when I stand up 2 I experience moderate orthostatic symptoms when I stand up and sometimes have to sit back down for relief 3 I experience severe orthostatic symptoms when I stand up and frequently have to sit back down for relief 4 I experience severe orthostatic symptoms when I stand and regularly faint if do not sit back down |
|
Conditions under which orthostatic symptoms occur 0 I never or rarely experience orthostatic symptoms under any circumstances 1 I sometimes experience orthostatic symptoms under certain conditions, such as prolonged standing, a meal, exertion (e.g., walking), or when exposed to heat (e.g., hot day, hot bath, hot shower) 2 I often experience orthostatic symptoms under certain conditions, such as prolonged standing, a meal, exertion (e.g., walking), or when exposed to heat (e.g., hot day, hot bath, hot shower) 3 I usually experience orthostatic symptoms under certain conditions, such as prolonged standing, a meal, exertion (e.g., walking), or when exposed to heat (e.g., hot day, hot bath, hot shower) 4 I always experience orthostatic symptoms when I stand up; the specific conditions do not matter |
|
Activities of daily living 0 My orthostatic symptoms do not interfere with activities of daily living (eg, work, chores, dressing, bathing) 1 My orthostatic symptoms mildly interfere with activities of daily living (eg, work, chores, dressing, bathing) 2 My orthostatic symptoms moderately interfere with activities of daily living (eg, work, chores, dressing, bathing) 3 My orthostatic symptoms severely interfere with activities of daily living (eg, work, chores, dressing, bathing) 4 My orthostatic symptoms severely interfere with activities of daily living (eg, work, chores, dressing, bathing). I am bed-bound or wheelchair-bound because of my symptoms |
|
Standing time 0 On most occasions, I can stand as long as necessary without experiencing orthostatic symptoms 1 On most occasions, I can stand more than 15 min before experiencing orthostatic symptoms 2 On most occasions, I can stand 5–14 min before experiencing orthostatic symptoms 3 On most occasions, I can stand 1–4 min before experiencing orthostatic symptoms 4 On most occasions, I can stand less than 1 min before experiencing orthostatic symptoms |
Impact of OH
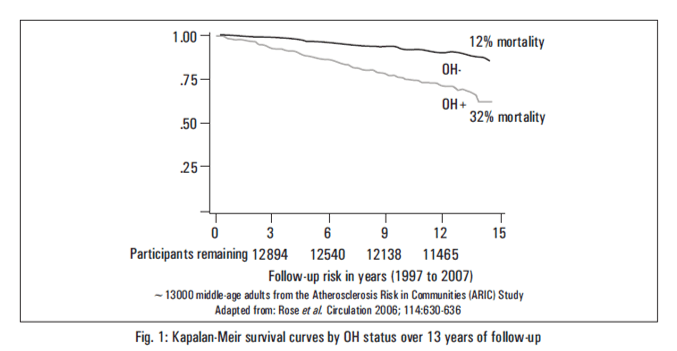
Current clinical and epidemiological studies show that OH is associated with syncope, falls and hospitalization. OH is an independent risk factor for cardiovascular disease, kidney disease and death. Several recent large studies have added to the strength of the evidence that OH at baseline is an independent risk factor for atrial fibrillation, stroke, congestive heart failure, kidney failure and excess mortality (Table 7). Orthostatic hypertension seems to increase risk for mortality.21-26
High Mortality
- Stronger association with mortality in population < 65 years
- 2-fold higher risk of death in individuals < 42 years of age
|
Author (Reference) |
n Age (Years) |
Prevalence OH (%) |
Follow up (Years) |
Morbidity /Mortality (Mean or Range) |
Comments |
|
Masaki et al. |
3522 71-93 |
6.9 |
4 |
SBP OH: RR 1.80 (95% CI:1.17–2.75) DBP OH: RR 1.52 (95% CI:1.01–2.29) for all-cause mortality |
OH was a significant independent predictor of all-cause mortality. |
|
Eigenbrodt et al. |
11,707 53.7 |
4.1 |
7.9 |
HR: 2.0 (95%CI:1.2–3.2) for ischemic stroke |
OH was predictive of ischemic stroke. |
|
Rose et al. |
12,433 54 |
4.9 |
6 |
HR 1.88 (95% CI: 1.32–2.67) for CHD |
There was an increased risk of developing CHD in patients with OH |
|
Luukinen et al. |
792 76 |
30 |
3.58 |
DBP at ≥8mmHg drop: HR: 2.91 (95% CI: 1.69–4.99) for MI; DBP ≥8mmHg drop: HR 2.10 (95% CI: 1.35–3.25) for mortality |
Only OH DBP ≥8 mmHg but not SBP drop was a significant predictive risk factor for MI or mortality. |
|
Rose et al. |
13,152 57 |
5.1 |
13 |
HR : 2.04 (95% CI: 1.57–2.66) for CVD HR: 2.12 (95% CI: 1.61–2.80) for non -cancer related cause deaths |
One-third of those with OH died over the 13 years of follow-up compared with 12% of those without OH. |
|
Weiss et al. |
471 81.7 |
34.2 |
3.47 |
No difference in mortality. |
OH did not predict all-cause and cause-specific mortality. |
|
Verwoert et al. |
5,064 68.1 |
18 |
6 |
HR: 1.31 (95% CI: 1.08–1.57) for CHD mortality. HR: 1.22 (95% CI: 1.09 to1.36) for all-cause mortality. |
OH increased the risk of CHD. |
|
Fedorowski et al. |
33, 346 45.7 |
6.2 |
22.7 |
SBP fall ≥30 mmHg: HR: 1.6 (95% CI: 1.3-1.9) for mortality, and HR: 1.6 (95% CI: 1.2-2.1) for coronary events. DBP fall ≥15 mmHg; HR: 1.4, (95% CI: 1.1- 1.9) for mortality, and HR: 1.7 (95% CI: 1.1-2.5) for coronary events. |
Mortality and coronary events risks were distinctly higher in those with SBP and DBP OH. |
|
OH: Orthostatic Hypotension, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, RR: Relative risk, HR: Hazard ratio, CHD: Coronary heart disease, CI: Confidence interval, MI: Myocardial infarction |
|||||
OH – Impaired Quality of Life
OH is associated with an increased rate of hospitalization (0.43%), syncope (4-15% and up to 30% in elderly) and a 2 fold increase in falls in elderly.27
Diagnosis
History and Physical Examination: Clues to Diagnosis of OH
There are no uniform recommendations currently, regarding when OH assessments must be performed. But it may be prudent to evaluate OH in patients with symptoms (such as dizziness, syncope/near syncope, falls) or risk factors (hypertensive medications).28
Most patients with significant OH may have no symptoms or they may have atypical symptoms. Hence a negative response to a question like 'Do you get dizzy or light-headed when you stand up' is not considered to be adequate screening for OH.19
The history must lay emphasis on when the OH symptoms began and on the circumstances when OH was first identified (recent illness, new medications or hospitalization). As per the definition, initial OH occurs within 3 min of standing, but in clinical practice, there are patients who can have initial OH within just 15 s of standing or they can have OH (beyond 3 min). Hence a clear description of the onset of symptoms is critical.19
The functional impact of OH and self-treatment measures (such as bending over or squatting) should be investigated. Medical and surgical problems, especially related to cardiovascular (hypertension or syncope) and neurological disorders must be investigated by the clinician (Table 7).19
Bedside Evaluation of the Autonomic Nervous System
Bedside evaluation of the autonomic nervous system consists primarily of observations of: cardiovascular reflexes, pupillary changes, and casual sweating patterns.29
Cardiovascular Reflexes
Evaluate the postural response of the BP and pulse rate. The patient is kept in the supine position for at least 2 minutes before BP and pulse rate are recorded. BP must be recorded at 2-minute intervals upon standing for 8 to 10 minutes or until symptoms occur. Patients must be kept under careful observation during this exercise in order to prevent hypotension to the point of syncope. Assistance must be readily available to prevent injury to the patient due to falling. If syncope occurs, the patient is immediately placed in the head down, legs elevated position to restore cerebral perfusion.29
Heart rate changes are recorded by a continuous rhythm strip on a conventional electrocardiogram obtained for 30 seconds prior to and 60 seconds after standing. In normal subjects maximal reflex acceleration of heart rate is seen in about 15 seconds after standing and it slows to near-supine rate by about 30 seconds after standing. If the heart rate does not increase with the development of symptomatic orthostatic hypotension is suggestive of autonomic dysfunction.26
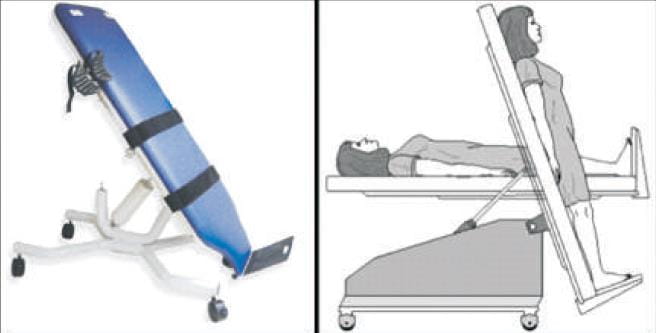
Indication and Procedure for Head-up Tilt –Table Testing
The Head-Up Tilt-Table Testing test is helpful in patients with a high probability of orthostatic hypotension despite an initial negative evaluation (e.g., Parkinson disease). The procedure is generally considered safe, but serious adverse events such as syncope and arrhythmias have been reported by some researchers and clinicians. All personnel involved in performing tilt-table testing must undergo training in advanced cardiac life support and resuscitation equipment must be readily available.12,29
Procedure
The tilt-table testing is performed in a quiet room with a temperature of 20°C to 24°C. The patient must rest in the supine position for five minutes before testing is started. Regular measurement and chronicling of heart rate and blood pressure are done.
The table must be slowly elevated to an angle between 60 to 80 degrees for three minutes.12,29
Interpretation of Results
Four common abnormal patterns can be seen in response to tilt-table testing (Table 8)12. The test may help
in differentiating orthostatic hypotension from other disorders that can present with symptoms of orthostasis, such
as neuro-cardiogenic syncope. Current data indicates that the sensitivity of tilt-table ![]() testing for diagnosing neuro-cardiogenic syncope about 65 percent, and specificity is as high as 100
percent.
testing for diagnosing neuro-cardiogenic syncope about 65 percent, and specificity is as high as 100
percent.
Positive test: If systolic blood pressure falls 20 mm Hg below baseline or if diastolic blood pressure falls 10 mm Hg below baseline, the test is considered to be positive. If symptoms occur during testing, the patient should be returned to the supine position immediately12.
|
Condition |
Physiologic response |
|
|
Normal |
Heart rate increases by 10 to 15 beats per minute DBP increases by 10 mm Hg or more |
|
|
Dysautonomia |
Immediate and continuing drop in SBP and DBP No compensatory increase in heart rate |
|
|
Neurocardiogenic syncope |
Symptomatic, sudden drop in BP Simultaneous bradycardia Occurs after 10 minutes or more of testing |
|
|
Orthostatic hypotension |
SBP decreases by 20 mmHg or more OR DBP decreases by 10 mmHg or more |
|
|
Postural orthostatic tachycardia syndrome |
Heart rate increases by at least 30 beats per minute |
|
The evaluation of suspected OH requires identification of reversible causes and underlying associated medical conditions as listed in the table below. The historical features listed suggest a specific diagnosis in the patient with OH. In addition to assessing for symptoms of orthostasis, the physician must investigate symptoms of autonomic dysfunction involving the gastrointestinal and genitourinary systems (Table 9).12
Differential Diagnosis of OH
There are many disorders which must be considered in the differential diagnosis of OH as listed in the Table 9 given below.12
|
Common |
|
|
Neurologic
|
|
Cardiovascular/Pulmonary
|
|
Endocrine/Renal
|
|
Other
|
Treatment
Acute OH often resolves with appropriate treatment of the underlying cause. Patients with chronic OH may benefit with non- pharmacologic and pharmacologic treatments. All patients with chronic OH require education regarding their diagnosis and goals of treatment. The chief goals of treatment include improving orthostatic BP without excessive supine hypertension, improving standing time and relieving orthostatic symptoms, improving presyncopal symptoms, improving the functional status and preventing syncope, falls, and related morbidity.30
The European Federation of Neurological Societies issued guidelines for the diagnosis and management of OH. Treatment must be initiated in a structured and stepwise approach. The first step is initiating nonn pharmacological interventions (e.g., lifestyle modifications and physical counter manoeuvres), and then adding pharmacological interventions (e.g., midodrine, droxidopa, fludrocortisone) as required in patients with severe OH.30
Non-pharmacological Measures
Nonpharmacological treatment must be advised to all patients as a part of the initial treatment protocol (Table 10).12,30
Avoidance of factors that may induce OH is recommended especially in mild forms. Several drug classes such as antihypertensives, antidepressants, sedatives, etc. are associated with OH. Whenever possible, the implicated drugs should be discontinued and replaced with alternative.31 The screening and monitoring of orthostatic hypotension should be instituted as a routine precaution in appropriately selected patients. Education of both the patients and caregivers regarding the mechanisms of OH is important.12
The next step is to initiate a range of non-pharmacological strategies. Patients must be advised to move to head - up position slowly, sit on the edge of the bed for some minutes after recumbence and activate calf muscles while supine. Physical counter manoeuvres can be started immediately at the onset of presyncopal symptoms. These include bending over forward to reduce the orthostatic difference between the heart and brain and compress the splanchnic vessels by increasing abdominal pressure, leg crossing with tension of the thigh, buttock and calf muscles (party position) and squatting to reduce blood pooling are effective in temporarily reducing OH. Not all patients can perform these manoeuvres. Elastic stockings and abdominal compression bands reduce venous pooling may be useful in some patients. Elevation of the head - end of the bed (20 – 30 cm) while sleeping, particularly in combination with low dose fludrocortisone, improves OH. A liberal intake of salt, at least 8 g (150 mmol) of sodium chloride daily, if needed as salt tablets (starting dose 500 mg t.i.d.), are recommended in order to compensate for renal salt loss. Water repletion (2 – 2.5 L/day) is vital. About 500 mL of water is effective in causing a rise in BP immediately. Cardiac pacing is not recommended in neurogenic OH.
If the probable medications implicated in causing OH cannot be discontinued, then patients should be instructed to take them at bedtime when possible, especially the antihypertensive drugs.
|
Discontinue medications that exacerbate OH
|
|
Adequate hydration
|
|
Physical countermeasures to decrease venous pooling
These simple manoeuvres can provide instantaneous improvement in orthostatic symptoms and tolerance. |
|
Isometric, lower-extremity physical exercise Devices to Decrease Venous Pooling
|
|
Patient education to increase central volume
|
|
Other important pointers to aid in the management of OH
|
Pharmacologic Measures
If non-pharmacologic measures do not adequately improve the symptoms of OH, then initiation of pharmacotherapy is indicated. For patients who are experiencing syncope, near-syncope, or falls, some clinicians believe that institution of pharmacotherapy at the outset of management may be appropriate. Clinicians must individualise treatment based upon the severity of the symptoms (Figure 3 and Table 11).12,14,32
|
Single Agents
|
|
Adrenergic Agonists and Sympathomimetics (prescribed as a PRN indication rather than at fixed intervals)
|
|
Splanchnic Vasoconstrictor
|
|
Vasopressor/Cardiac Therapy
|
|
Combination Therapy
|
Midodrine in OH
Midodrine was approved by the US Food and Drug Administration (FDA) in 1996 for OH.
Mechanism of action
Midodrine is a prodrug. After oral ingestion, midodrine undergoes enzymatic hydrolysis to an active metabolite, desglymidodrine which is a pure alpha-1 agonist. It causes activation of the alpha-adrenergic receptors of the arteriolar and venous vasculature, resulting in an increase in vascular tone and elevation of BP.32
Desglymidodrine does not stimulate cardiac beta-adrenergic receptors. Desglymidodrine diffuses poorly across the blood-brain barrier and hence it is not associated with effects on the central nervous system. Administration of midodrine results in a rise in standing, sitting, and supine SBP and DBP in patients with OH of varied etiologies. Standing SBP is elevated by 15 to 30 mmHg at 1 hour after a 10-mg dose of midodrine, with some persisting effects observed for 2 to 3 hours. Current data indicates that midodrine has no clinically significant effect on standing or supine pulse rates in patients with autonomic failure.34,35
Pharmacokinetics of Midodrine
Midodrine is rapidly absorbed and the absolute bioavailability of midodrine (measured as desglymidodrine) is 93%. The plasma levels of the prodrug peak after about half an hour. During metabolism, deglycination of midodrine to desglymidodrine takes place. Midodrine undergoes insignificant renal elimination. The renal clearance of desglymidodrine is of the order of 385 mL/minute, most, about 80%, by active renal secretion. The half –life of midodrine is 25 minutes. The metabolite reaches peak blood concentrations about 1 to 2 hours after a dose of midodrine and has a half-life of about 3 to 4 hours.36
Dosing and Administration
Midodrine use has been well-tolerated at 2.5 mg, 5 mg and 10 mg oral doses. Typical dosing is between 2.5 and 15 mg once to three times daily during waking hours. The dose is typically up-titrated to symptomatic relief.32
Warnings
Supine Hypertension: The most potentially serious adverse reaction associated with midodrine therapy is marked elevation of supine arterial BP (supine hypertension). Systolic pressure of about 200 mmHg was observed in about 13.4% of patients given 10 mg of Midodrine. This rise in BP was more likely to be observed in patients with elevated pre-treatment SBP (mean 170 mmHg). Currently there is no data in patients with initial supine systolic pressure above 180 mmHg. Use of Midodrine in such patients is not recommended. Sitting BP were also elevated by midodrine therapy. It is recommended that supine and sitting BP must be monitored in patients maintained on midodrine.37 Uncontrolled hypertension raises the risk of cardiovascular events, especially stroke.38
Precautions: General
- Supine hypertension: The potential for supine and sitting hypertension must be evaluated at the time of initiation of midodrine therapy. Supine hypertension can often be controlled by preventing the patient from becoming fully supine, i.e., sleeping with the head of the bed elevated. The patient should be cautioned and educated to report to the physician any symptoms of supine hypertension immediately. Symptoms may include pounding in the ears, headache, blurred vision, etc. If supine hypertension persists, the patient should be advised to discontinue the medication immediately.39
- In order to reduce the potential for supine hypertension during sleep, midodrine should not be given after the evening meal or less than 4 hours before bedtime. Total daily doses greater than 30 mg have been tolerated by some patients, but their safety and usefulness have not been studied systematically or established. Because of the risk of supine hypertension, midodrine should be continued only in patients who appear to attain symptomatic improvement during initial treatment.40
- A slight slowing of the heart rate may occur after administration of midodrine, primarily due to vagal reflex.41
- Cautious use is recommended when midodrine is used concomitantly with cardiac glycosides (such as digitalis), psychopharmacologic agents, beta-blockers or other agents that directly or indirectly reduce heart rate.39
- Patients who experience any signs or symptoms suggesting bradycardia (pulse slowing, increased dizziness, syncope, cardiac awareness) must be advised to discontinue midodrine and should be re-evaluated.39
- Midodrine should be used cautiously in patients with urinary retention problems, as desglymidodrine acts on the alpha-adrenergic receptors of the bladder neck.39
- Midodrine should be used with caution in OH patients with comorbid diabetes as well as in patients with a history of visual problems who are also taking fludrocortisone acetate, which can an increase intraocular pressure and cause glaucoma.42
Contraindications
Midodrine is contraindicated in patients with severe organic heart disease, acute renal disease, urinary retention, pheochromocytoma or thyrotoxicosis. Midodrine must not be used in patients with persistent and excessive supine hypertension.43
Drug Interactions
When administered concomitantly with midodrine, cardiac glycosides may enhance or precipitate bradycardia, A.V. block or arrhythmia. The risk of hypertension increases with concomitant administration of drugs that increase BP (phenylephrine, pseudoephedrine, ephedrine, dihydroergotamine, thyroid hormones, or droxidopa).39
Avoid concomitant use of drugs that increase BP. If concomitant use cannot be avoided, monitor BP closely. Avoid use of MAO inhibitors or linezolid with midodrine. If midodrine is used in patients concomitantly treated with fludrocortisone acetate with or without salt supplementation, the potential for supine hypertension must be considered and the patients must be monitored carefully.43
The risk of supine hypertension can be minimised by either reducing the dose of fludrocortisone acetate or decreasing the salt intake before initiation of treatment with midodrine. The effects of midodrine are antagonised by alpha-adrenergic blocking agents, such as prazosin, terazosin and doxazosin.44There is no supporting evidence, that the high renal clearance of desyglymidodrine (a base) is due to active tubular secretion by the base-secreting system also responsible for the secretion of drugs such metformin, cimetidine, ranitidine, procainamide, triamterene, flecainide and quinidine. There may be a potential for drug-drug interactions with these drugs.45
Adverse reactions
The most frequent adverse reactions reported in clinical trials were supine and sitting hypertension; paresthesia and pruritus, mainly of the scalp; goose bumps; chills; urinary urge; urinary retention and urinary frequency.42
Less frequent adverse reactions were headache; feeling of pressure/fullness in the head; vasodilation/flushing face; confusion/thinking abnormality; dry mouth; nervousness/anxiety and rash. Other adverse reactions that occurred rarely were visual field defect; dizziness; skin hyperesthesia; insomnia; somnolence; erythema multiforme; canker sore; dry skin; dysuria; impaired urination; asthenia; backache; pyrosis; nausea; gastrointestinal distress; flatulence and leg cramps.
The chief potentially serious adverse reaction associated with midodrine therapy is supine hypertension. The feelings of paresthesia, pruritus, piloerection and chills are pilomotor reactions associated with the action of midodrine on the alpha-adrenergic receptors of the hair follicles. Feelings of urinary urgency, retention and frequency are associated with the action of midodrine on the alpha-receptors of the bladder neck.46
Clinical Experience with Midodrine
Clinical benefit of midodrine hydrochloride in symptomatic OH: a double-blind, placebo-controlled, randomized, tilt-table study10
Midodrine hydrochloride is a short-acting pressor agent that raises blood pressure in the upright position in patients with OH. A post marketing study of midodrine was conducted to confirm clinical benefit of midodrine in OH.
Aim
The purpose of this study was to evaluate the clinical benefit of midodrine with regard to symptom response.
Study design
Double-blind, placebo-controlled, randomized, crossover, multicenter study.
|
|
Midodrine |
Placebo |
||
|
The least-squares mean time to syncopal symptoms or near-syncope after tilt-table initiation (mean ± standard error) |
1626.6 ± 186.8 s |
1105.6 ± 186.8 s
P = 0.0131 |
||
Study features
Thirty three patients (18 years) with severe symptomatic OH and treated with a stable dose of midodrine for at least 3 months. The patients’ first daily dose of midodrine ranged from 2.5 mg to15 mg.
Observations
The least-squares mean time to syncopal symptoms or near-syncope after tilt-table initiation was significantly longer in the midodrine treated group as compared to the placebo treated group.
Adverse events were mild or moderate in severity.
Conclusion
Midodrine was effective treatment for symptomatic OH and was well-tolerated.
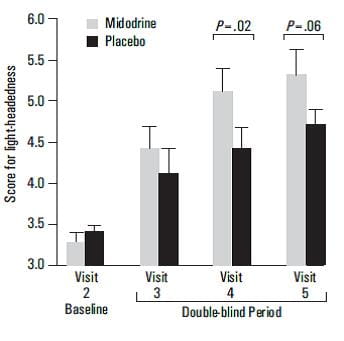
Efficacy of Midodrine vs Placebo in Neurogenic OH19
Objective
To evaluate the efficacy of a 10-mg dose of midodrine 3 times per day in improving standing BP and ameliorating symptoms of OH in patients with neurogenic OH.
Design
One hundred seventy one patients with OH participated in a multicenter, randomized, placebo-controlled study.
Patient Population
The most common diagnostic groups were
- Multiple system atrophy (Shy-Drager syndrome, n=40)
- Pure autonomic failure (Bradbury-Eggleston syndrome, n=37)
- Diabetic autonomic neuropathy (n=37)
The remaining patients had
- Parkinsonism (n=19)
- Miscellaneous disorders (n=29)
The majority of patients classified as having miscellaneous disorders are listed under 3 major categories of orthostatic intolerance (postural tachycardia syndrome, OH with neurocardiogenic syncope, mitral valve prolapse), autonomic neuropathy, and neurologic disease (olivopontocerebellar atrophy, spinal cord injury, syringomyelia).
Treatment Groups
One hundred and seventy one patients were randomized to a 10-mg dose of midodrine or placebo 3 times per day in a 6-week study, comprising single-blind run-in (at week 1) and washout at weeks 5 and 6, with an intervening double-blind period (weeks 2 to 4).
Main outcome measures
The primary end points were improvement in standing SBP, symptoms of light-headedness and a global symptom relief score (by the investigator and patient separately).
Results
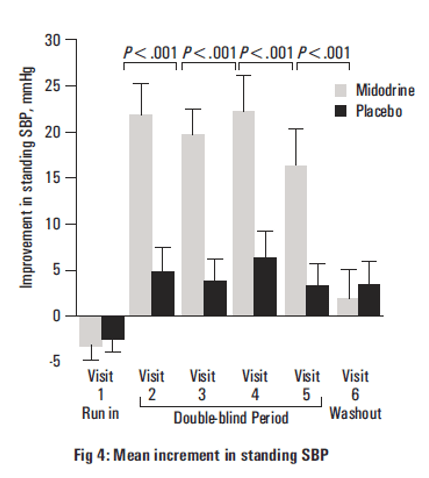
- Midodrine caused improvements in standing SBP at all time points (P<0.001 at visits 2,3,4, and 5).
- Midodrine improved reported symptoms by the end of the second week of treatment (P=.001).
Midodrine significantly improved the global symptom relief score as rated by both the patient (P=0.03) and the investigator (P<0.001).
The main adverse effects were those of pilomotor reactions, urinary retention and supine hypertension.
Conclusion
Midodrine is efficacious and safe in the treatment of neurogenic OH.
Comparison of the Efficacy of Midodrine with Servo-Controlled Splanchnic Venous Compression in the Treatment of OH47
Background
Patients with severe impairment of autonomic pathway have disabling OH. There are only two drugs approved for the treatment of neurogenic OH, the aa-1 agonist midodrine and the norepinephrine prodrug droxidopa.
Venous return can be improved in these patients with the compression of venous capacitance beds with lower body compression garments and can be useful in treating OH. The chief advantages of this approach include immediate pressor responses which could be achieved only when required (i.e., standing); and it can be safely combined with virtually any drug therapy.
Aim
To evaluate the efficacy of combination of venous binder with midodrine in patients with autonomic failure.
Study design
Single-blind, crossover study.
Patient population and treatment
Nineteen patients with autonomic failure were randomized to receive either placebo, midodrine (2.5-10 mg), or placebo combined with binder on separate days.
Observations
Eight patients received a single dose of 10 mg of midodrine, ten patients 5 mg and one patient 2.5 mg. The compression level of 40 mmHg was well-tolerated in all patients except for one, in whom a lower compression level (20 mmHg) was used. Average baseline seated SBP was similar among treatment groups suggesting that no significant carryover effects were present between treatment groups.
|
|
Placebo binder |
Placebo with |
Midodrine |
|||
|
Baseline seated BP |
105±6 mmHg |
111±5 mmHg |
105±4 mmHg; P=0.338 |
|||
One hour after drug administration, midodrine significantly increased seated SBP compared to placebo (P<0.001.) and to placebo and binder (P=0.017).
|
|
Midodrine |
Placebo |
Placebo + Binder |
|||
|
Increase in seated SBP one hour after drug administration |
31±5 mmHg |
9±4 mmHg P<0.001 |
7±5 mmHg P=0.017 |
|||
|
Number of patients who were able to stand for the full 10 minutes of the post-intervention orthostatic test |
14 |
10 |
14 |
|||
|
The increase in 1–minute standing SBP from pre-intervention |
16±3 mmHg |
5±4 mmHg |
12±4 mmHg |
|||
On standing, inflation of the abdominal binder and midodrine caused a similar significant increase in AUCSBP (i.e. improved orthostatic tolerance) in comparison with placebo (195±35 and 197±41 vs. 19±38 mmHg*min on the placebo day, P=0.019 and P=0.010 respectively. An increase in SBP of 19.5 mmHg over the 10-minute standing period for the binder group vs. 1.9 mmHg for the placebo group was observed.
Orthostatic symptom scores were recorded at two time points namely -at baseline and 1 hour after placebo (n=18), placebo and binder (n=17), and midodrine (n=18). The total orthostatic symptom scores after 1 hour post-intervention significantly reduced with inflation of the abdominal binder and with midodrine but this response was not observed to occur with placebo.
|
Orthostatic symptom scores |
Abdominal binder |
Midodrine |
Placebo |
|||
|
At baseline |
21.9± 3.6 |
25.6±3.4 |
19.6±3.5 |
|||
|
After 1 hour |
16.3±3.1 |
14.2±3.2 |
0.1±3.3 |
|||
|
|
P=0.032 |
P<0.001 |
P=0.756 |
|||
The combination of midodrine and binder compared with midodrine alone
The combination of midodrine and binder caused a greater increase in orthostatic tolerance (AUCSBP, 326±65 vs. 140±53 mmHg*min for midodrine alone, P=0.028, n=21), and decreased orthostatic symptoms (from 21.8±3.2 to 12.9±2.9, P<0.001).
Conclusion
In the management of neurogenic orthostatic hypotension (nOH), midodrine has an efficacy comparable to servo-controlled abdominal venous compression with an automated inflatable binder. The combination of therapies produces greater improvement in orthostatic tolerance.
Double-blind, dose-response study of midodrine in neurogenic OH48
Aim
To determine the best therapeutic strategy for the use of midodrine in patients with nOH.
Study Design
Double-blind, placebo-controlled, four-way crossover trial.
Patient Population
Twenty five patients with nOH were randomized to receive placebo or midodrine 2.5, 10 or 20 mg.
Observations
Midodrine caused a significant increase in standing SBP. A linear dose response relation of midodrine was observed.
Conclusion
A 10-mg dose of midodrine two to three times daily effectively increased orthostatic BP and relieved symptoms in patients with nOH.
Efficacy of midodrine in orthostatic hypotension due to autonomic failure49
Background
Autonomic failure is a known cause of OH. Midodrine has been approved for the management of OH.
Study Design
Double-blind, placebo-controlled study.
Patient Population
Ninety seven patients (mean age 61 years) with OH.
Treatment Groups
Placebo or Midodrine at a dose of 2.5 mg, 5 mg, or 10 mg was administered three times a day for 4 weeks.
The patients receiving 5 mg or 10 mg of midodrine were administered doses that were increased at weekly intervals by 2.5-mg increments until the designated dose was reached.
Observations
An increase in standing SBP of 22 mmHg was observed with midodrine (10 mg) (28%, P< 0.001 versus placebo).
Midodrine caused a significant improvement in the symptoms of weakness/fatigue, syncope, low energy level, dizziness/light-headedness, impaired ability to stand and feelings of depression (P < 0.05).
Conclusion
In patients with moderate-to-severe OH due to autonomic failure, midodrine is effective and well tolerated.
Abbreviations
ACEI: Angiotensin Converting Enzyme Inhibitors
BP: Blood pressure
CHD: Coronary heart disease
CI: Confidence interval
DBP: Diastolic blood pressure
ECG: Electrocardiography
FDA: Food and Drug Administration
Hb: Hemoglobin
HR: Hazard ratio
MI: Myocardial infarction
MIBG: Metaiodobenzylguanidine
nOH: Neurogenic orthostatic hypotension
OH: Orthostatic Hypotension
PET: Poly ethylene terephthalate
RR: Relative risk
SBP: Systolic blood pressure
References
1. Mader SL. Orthostatic hypotension in older patients. Aging Health. 2006; 2: 54–61.
2. Mukai S, Lipsitz LA. Orthostatic hypotension. Clin. Geriatr. Med. 2002; 18(2):253–268.
3. Jansen RW. Postprandial hypotension: simple treatment but difficulties with the diagnosis. J. Gerontol. A Biol. Sci. Med. Sci. 2005; 60(10):1268–1270.
4. Sorond FA, Khavari R, Serrador JM, et.al. Regional cerebral autoregulation during orthostatic stress: agea related differences. J. Gerontol. A Biol. Sci. Med. Sci. 2005; 60(11): 1484–1487.
5. Van Beek AH, Sijbesma JC, Jansen RW, et al. Cortical oxygen supply during postural hypotension is further decreased in Alzheimer's disease, but unrelated to cholinesterase-inhibitor use. J. Alzheimers. Dis. 2010; 21(2): 519–526.
6. Weiss A, Grossman E, Beloosesky Y, et al. Orthostatic hypotension in acute geriatric ward. Is it a consistent finding? Arch. Int. Med. 2002; 162(20): 2369–2374.
7. Zhou Y, Ke SJ, Qiu XP, et al. Prevalence, risk factors, and prognosis of orthostatic hypotension in diabetic patients: A systematic review and meta-analysis.Medicine (Baltimore). 2017; 96(36):e8004.
8. Perlmuter LC, Sarda G, Casavant V, et al. A review of the etiology, associated comorbidities, and treatment of orthostatic hypotension. Am J Ther. 2013; 20(3):279-91.
9. Smith W, Wan H, Much D, et al. Clinical benefit of midodrine hydrochloride in symptomatic orthostatic hypotension: a phase 4, double-blind, placebo-controlled, randomized, tilt-table study. Clin Auton Res. 2016; 26(4):269-77.
10. Byun JI, Moon J, Kim DY, et al. Efficacy of single or combined midodrine and pyridostigmine in orthostatic hypotension. Neurology. 2017; 89(10):1078-1086.
11. Arnold AC, Raj SR. Orthostatic Hypotension: A Practical Approach to Investigation and Management. Can J Cardiol. 2017; 33(12):1725-1728.
12. Lainer JB, Mote MB, Clay EC. Evaluation and Management of Orthostatic HypotensionAm Fam Physician. 2011; 84(5):527-536.
13. Roy AG, Gopinath S, Kumar S, et al. Delayed Orthostatic Hypotension: A Pilot Study from India. Ann Indian Acad Neurol. 2017; 20(3):248-251.
14. Ricci F, Caterina RD, Fedorowski A. Orthostatic Hypotension Epidemiology, Prognosis, and Treatment. Journal of the American College of Cardiology. 2015; 66:7.
15. Metzler M, Duerr S, Granata R, et al. Neurogenic orthostatic hypotension: pathophysiology, evaluation, and management. J Neurol. 2012; 260(9):2212-9.
16. Baliga R, Prabhu G. Orthostatic hypotension in healthy elderly: Is it a myth? N Am J Med Sci. 2010; 2(9):416-8.
17. Menon AS, Dixit A, Garg MK, et al. Cardiac Autonomic Neuropathy in Patients with Type 2 Diabetes Mellitus at High Risk for Foot Ulcers. Indian J Endocrinol Metab. 2017; 21(2):282-285.
18. Damanti S, Consonni D, Valentini A, et al. Orthostatic hypotension, an often-neglected problem in community dwelling older people: discrepancies between studies and real life. J Geriatr Cardiol. 2018; 15(10):644-646.
19. Low PA, Gilden JL, Freeman R, et al. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group.JAMA. 1997; 277(13):1046-51.
20. Aung AK, Corcoran SJ, Nagalingam V, et al. Prevalence, associations, and risk factors for orthostatic hypotension in medical, surgical, and trauma inpatients: an observational cohort study. Ochsner J. 2012; 12(1):35-41.
21. Mussi C, Ungar A, Salvioli G, et al. Evaluation of Guidelines in Syncope Study 2 Group. J Orthostatic hypotension as cause of syncope in patients older than 65 years admitted to emergency departments for transient loss of consciousness. Gerontol A Biol Sci Med Sci. 2009; 64(7):801-6.
22. Shibao C, Grijalva CG, Lipsitz LA, et al. Early discontinuation of treatment in patients with orthostatic hypotension. Auton Neurosci. 2013; 177(2):291-6.
23. Fedorowski A, Melander O. Syndromes of orthostatic intolerance: a hidden danger. J Intern Med. 2013; 273(4):322-35.
24. Fedorowski A, Hedblad B, Melander O. Early postural blood pressure response and cause-specific mortality among middle-aged adults. Eur. J. Epidemiology. 2011; 26(7), 537–546.
25. Franceschini N, Rose KM, Astor BC, et al. hypotension and incident chronic kidney disease: the atherosclerosis risk in communities study. Hypertension. 2010; 56(6):1054-9.
26. Verwoert GC, Mattace-Raso FU, Hofman A, et al. Orthostatic hypotension and risk of cardiovascular disease in elderly people: the Rotterdam study. J Am Geriatr Soc. 2008; 56(10):1816-20.
27. Phelan EA, Mahoney JE, Voit JC, et al. Assessment and Management of Fall Risk in Primary Care Settings. Med Clin North Am. 2015; 99(2): 281–293.
28. Chobanian AV, Bakris GL, Black HR et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003; 42(6), 1206–1252.
29. Barbato AL. Bedside Evaluation of the Autonomic System. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition.
30. Joseph A, Wanono R, Flamant M, et al. Orthostatic hypotension: A review. Nephrol Ther. 2017; 13 Suppl 1:S55-S67.
31. Verhaeverbeke I, Mets T. Drug-induced orthostatic hypotension in the elderly: avoiding its onset. Drug Saf. 1997; 17(2):105-18.
32. Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension J Neurol. 2017; 264(8): 1567–1582.
33. Parsaik AK, SinghB, Altaya O, et al. Midodrine for Orthostatic Hypotension: A Systematic Reviewand Metad Analysis of Clinical Trials. J Gen Intern Med 28(11):1496–503.
34. Singer W, Joyner MJ, Sandroni P, et al. Midodrine Efficacy in Orthostatic Hypotension. J Gen Intern Med. 2014; 29(11): 1440–1441.
35. Cheshire WP. Chemical pharmacotherapy for the treatment of orthostatic hypotension. Expert Opin Pharmacother. 2018; 30:1-13.
36. Wang B, Siahaan TJ, Soltero RA. Drug Delivery: Principles and Applications. John Wiley & Sons, 2005; Science: 464 pages.
37. Ferrar M, Chau WL, West J, et al. Prescribing Guidelines for Use of Midodrine for Orthostatic (Postural) Hypotension. The Sheffield area prescribing group. Adapted from: http://www.potsuk.org/UserFiles/File// Midodrine_Sheffield_Guidelines_2017.pdf. Last accessed on 28th December, 2018.
38. Zhou D, Xi B, Zhao M, et al. Uncontrolled hypertension increases risk of all-cause and cardiovascular disease mortality in US adults: the NHANES III Linked Mortality Study. Sci Rep. 2018; 8(1):9418.
39. Hale GM, Valdes J, Brenner M. A Review of the Treatment of Primary Orthostatic Hypotension. Annals of Pharmacotherapy 2017; 51 (5):417-428.
40. Midodrine for Orthostatic Hypotension. February 2011 from the National Electronic Library for Medicines. Adapted from: www.nelm.nhs.uk. Last accessed on 28th December, 2018.
41. Cornwall & Ios Health Community Shared Care Guideline. Adapted from: https://doclibrary- rcht.cornwall.nhs.uk/DocumentsLibrary/RoyalCornwall Hospitals Trust/ Clinical/Pharmacy/Midodrine.pdf. Last accessed on 28th December, 2018.
42. Gilden JL. 112 - Midodrine and Other Sympathomimetics. Primer on the Autonomic Nervous System (Second Edition) 2004; 413-415.
43. Ashley C and Currie A. The Renal Drug Handbook. Third Edition. Radcliffe Publishing Oxford, New York.
44. Jones & Bartlett Learning. 2012 Nurse's Drug Handbook. Jones & Bartlett Publishers, 2011; Medical: 1250 pages.
45. Somogyi A, McLean A, Heinzow B. Cimetidine-procainamide pharmacokinetic interaction in man: evidence of competition for tubular secretion of basic drugs. Eur J Clin Pharmacol. 1983; 25(3):339-45.
46. Orthostatic hypotension due to autonomic dysfunction: Midodrine. Evidence summary Published: 2015 nice.org.uk/guidance/esnm61.
47. Okamoto LE, Diedrich A, Baudenbacher FJ, et al. Efficacy of Servo-Controlled Splanchnic Venous Compression in the Treatment of Orthostatic Hypotension: A Randomized Comparison with Midodrine. Hypertension. 2016; 68(2):418-26.
48. Wright RA, Kaufmann HC, Perera R, et al. A double-blind, dose-response study of Midodrine in neurogenic orthostatic hypotension. Neurology. 1998; 51(1):120-4.
49. Jankovic J, Gilden JL, Hiner BC, et al. Neurogenic orthostatic hypotension: a double-blind, placebo-controlled study with Midodrine. Am J Med. 1993; 95(1):38-48.