Faropenem 200 Mg
Farobact
Fusion Power
Data Sheet
Faropenem is a new oral penem antibiotic which shows broad antibacterial activity against both aerobic and anaerobic gram-positive and gram-negative bacteria.
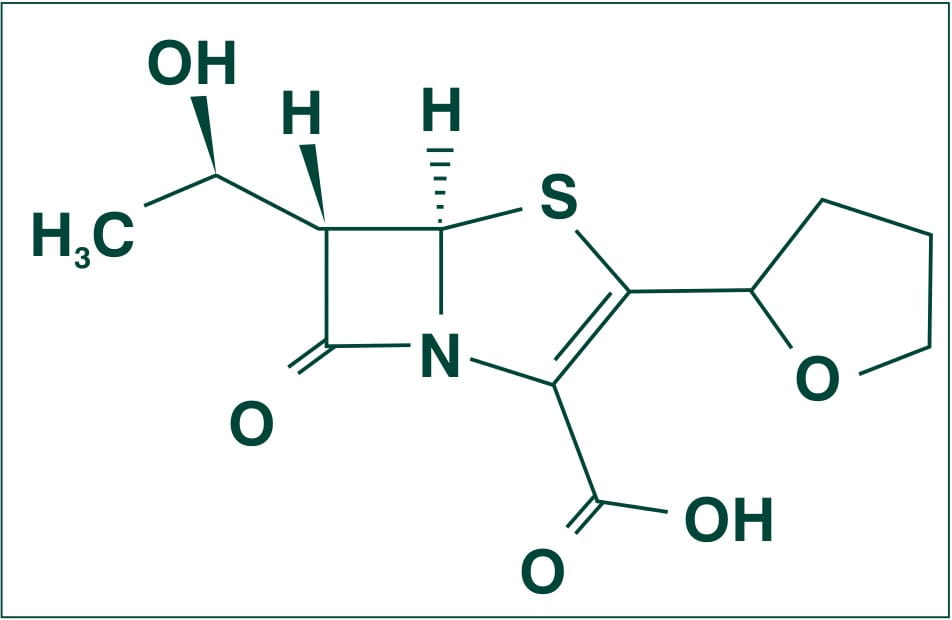
Chemically, Faropenem is described as Monosodium (5R, 6S)-6-[(IR)-l-hydroxyethyl]-7-oxo-3-[(2R)-tetrahydrofuran-2-yl]-4-thia-l-azabicyclo [3.2.0] hept-2-ene-2-carboxylate hemipentahydrate.
Its molecular weight is 352.34.
The empirical formula is C12H14NNaO5S. 2 ½ H2O, and its structural formula is:
Faropenem Sodium occurs as white to light yellow, crystals or crystalline powder. It is freely soluble in water and methanol, and slightly soluble in ethanol.1
Faropenem is bactericidal, it has a strong affinity for the high molecular penicillin binding proteins (PBPs) of the cell wall, which is essential for multiplication of bacilli and thus acts by inhibiting the cell wall synthesis. Faropenem shows broad antibacterial activity against both aerobic and anaerobic gram-positive and gram-negative bacteria. Faropenem is highly stable against various beta-lactamases and binds preferentially to the penicillin-binding proteins (PBPS) 2 and 1A of Escherichia coli.
Microbiology
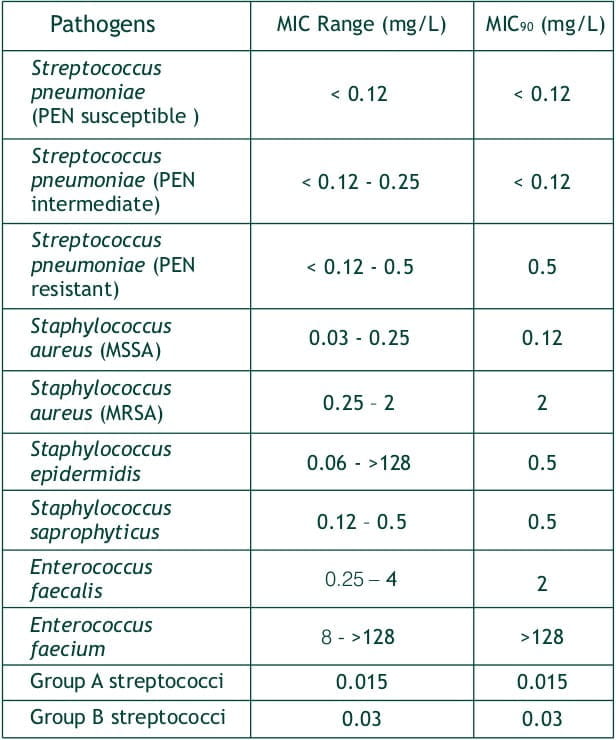
Faropenem was found to be active against gram positive, gram negative and anaerobic bacteria
- Enterococcus faecalis,
- Oxacillin-susceptible Staphylococci,
- Neisseria gonorrheae,
- Neisseria meningitides,
- Haemophillus influenzae,
- Moraxella catarrhalis,
- Streptococcus pyogenes,
- Staphylococcus saprophytics,
- Staphylococcus epidermidis,
- Group A Streptococci,
- Group B Streptococci,
- Streptococcus milleri,
- Streptococcus viridans,
- Staphylococcus aureus,
- Enterococcus faecalis,
- Enterococcus faecium,
- E.coli,
- Klebsiella spp.,
- Proteus mirabilis,
- Citrobacter spp.,
- Salmonella spp.,
- Shigella spp.,
- Providentia stuartii,
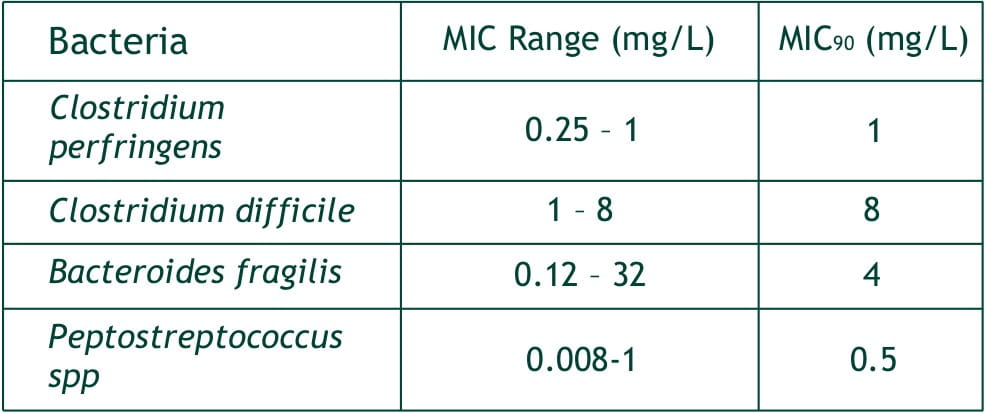
- Bacteroides fragilis,
- C. perfringens,
- Peptostreptococcus spp.
Faropenem was less active against the following:
- Citrobacter freundii,
- Proteus mirabilis,
- Enterobacter spp.,
- Proteus vulgaris and
- Morganella morganii.
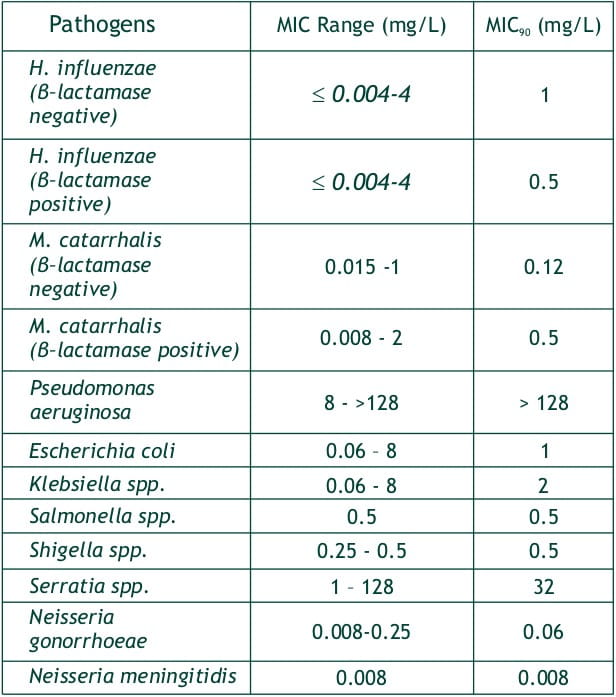
In Vitro Activity
Faropenem sodium
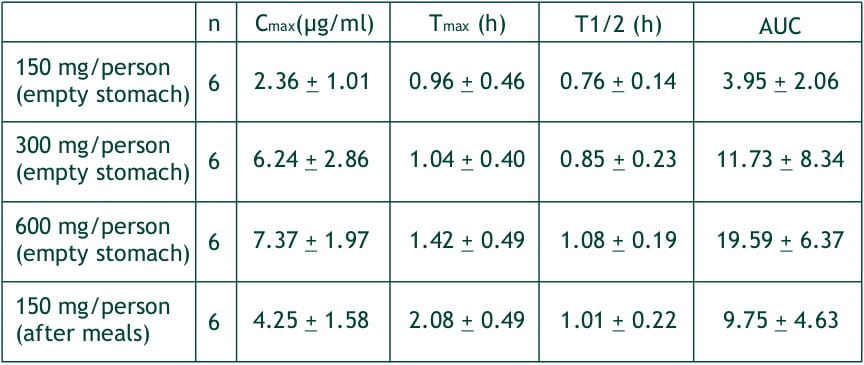
After single oral dose of faropenem in fasting healthy volunteers at 150, 300 and 600 mg, the plasma levels of faropenem reached Cmax, of 2.4, 6.2 and 7.4 (mg/ml, respectively at about 1-1.5 hours (Tmax). The AUCs of faropenem were 3.94, 11.73 and 19.59 ?g.h/ml. These Cmax and AUCs were proportional to the doses, and the respective urinary recoveries were 3.12, 6.78 and 5.26% of the dose. The half-life of faropenem is about 1 hour irrespective of the dosage quantity.
At a single dose of 300 mg in normal healthy adult after meals, the average Tmax, was delayed by about 1 hour, but Cmax AUC and urinary recovery were not different from those in the fasting state. In the multiple dosing study with 400 mg t.i.d., the Cmax on day 1, 4 and 7 (1st, 10th and 19th administration) were 5.5, 4.3 and 4.8 mg/ml, respectively. And the respective AUCs were 12.5, 10.1 and 12.2 mg.h/ml, demonstrating no cumulative effect.
Faropenem was found in the sputum of patients, fluid that oozes at the time of tooth extraction, tonsil tissues, maxillary sinus mucous membrane tissues, female genital organ tissues, eyelids, subcutaneous cell tissues and prostate tissues.
Before excretion in urine the absorbed faropenem gets metabolized by dehydropeptidase-l (DHP I) present in the kidney. The metabolites were found in blood and urine. The metabolites do not demonstrate antibacterial activity.
Faropenem is primarily excreted through kidneys and the rate of excretion in urine (0 ~ 24hrs) 150, 300 and 600 mg (given empty stomach to normal healthy adult) was 3.1 ~ 6.8% and the highest concentration in urine was 21.7, 55.6 and 151.5 mg/ml respectively in 0-2 hours and after 12 hours it was almost nil.
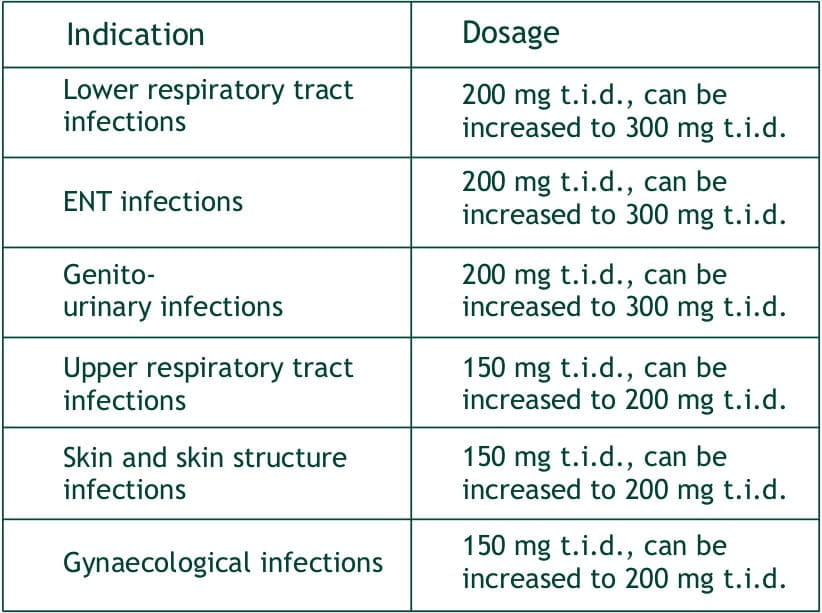
Faropenem sodium tablets are indicated in the treatment of the following infections:
Lower respiratory tract infections: e.g. acute bronchitis, pneumonia, pulmonary suppuration.
ENT infections: e.g. otitis externa, tympanitis, sinusitis
Genito-urinary infections : e.g. pyelonephritis, cystitis, prostatitis, seminal gland inflammation.
Upper respiratory tract infections: e.g. pharyngitis, tonsillitis.
Skin and skin structure infections : e.g pustular acne, folliculitis, contagious impetigo, erysipelas, lymphangitis, suppurative nail inflammation, subcutaneous abscess, hidradenitis (sweat gland inflammation), infective sebaceous cyst, chronic pyoderma, secondary infection of external wounds or surgical wound.
Gynaecological infections: e.g. adnexitis, bartholin gland inflammation.
Duration of treatment: The duration of treatment depends upon the severity of infection, clinical response and bacteriological findings.
Acute Bacterial Sinusitis
Objective: To compare efficacy and safety of 7-10 days course of 300 mg BID Faropenem medoxomil to a 10 day course of 250 mg BID cefuroxime axetil.
N=1106≥12years
Countries involved: Canada and USA
Results: Clinical cure
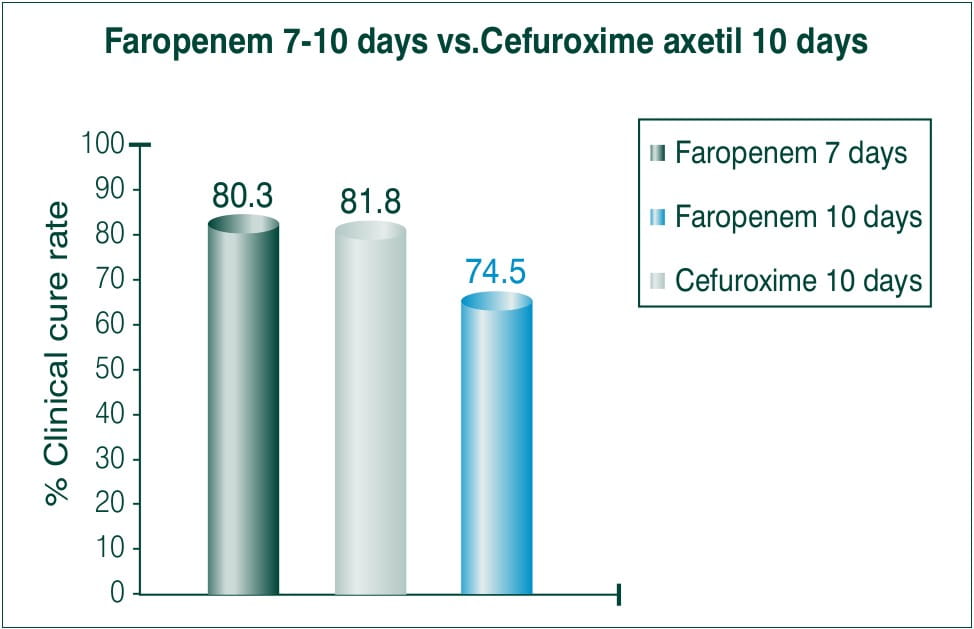
Conclusion: The treatment regimen of Faropenem both for 7 and 10 days BID was statistically superior to that of 10 days BID of cefuroxime axetil treatment.5
Acute Bacterial Sinusitis
Objective: To evaluate the efficacy and safety of 300 mg Faropenem medoxomil BID for 7 days vs. 250 mg cefuroxime axetil BID for 7days.
N = 548 subjects > 18 years of age
Countries involved: European and Israel
Results:
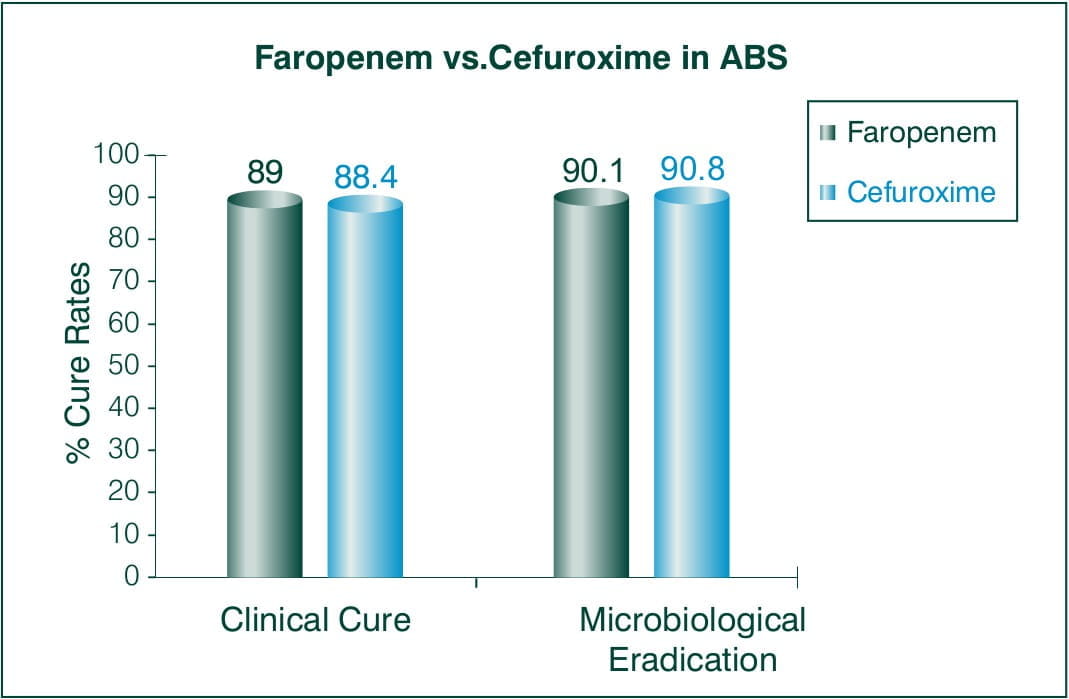
Conclusion: This study demonstrates the statistical non-inferiority of 7 days regimen of Faropenem to 7 day cefuroxime axetil regimen.5
Acute Exacerbation of Chronic Bronchitis (AECB)
Objective: To assesses the efficacy of Faropenem as a treatment for AECB 5 days BID 300 mg Faropenem medoxomil vs. 5 days BID 500 mg azithromycin on day 1 and 250 mg for remaining 2-5 days.
N = 824 patients
Countries involved: US and Argentina
Results:
Faropenem -300 mg BID, Azithromycin -500 mg QD (day 1), 250 mg QD (day2-5)
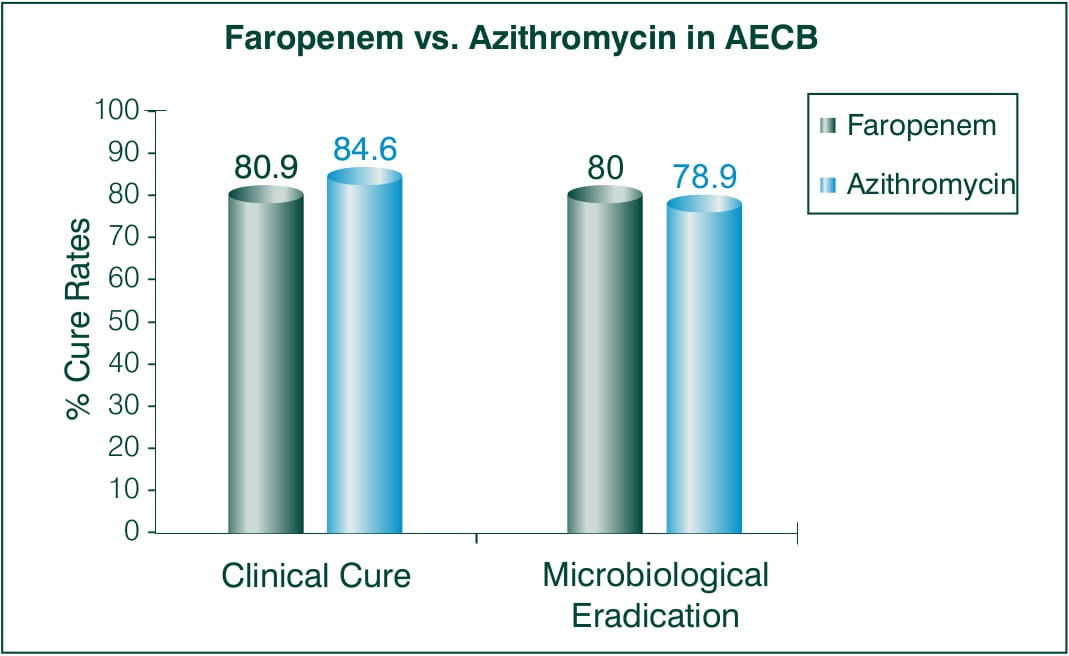
Conclusion: Faropenem medoxomil was as effective as the standard treatment regimen of azithromycin.5
Community Acquired Pneumonia1
Objective: To explore the efficacy and safety of Faropenem 200 mg t.i.d in patients diagnosed with community acquired pneumonia.
N =287 (between 14 and 88) years of age
Study conducted at Indraprastha Apollo Hospital, New Delhi
Results: Faropenem 200 mg t.i.d
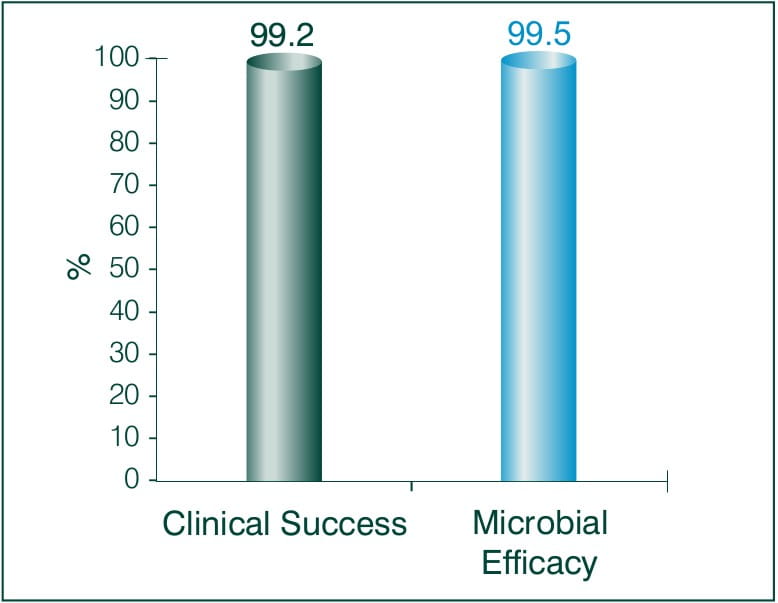
Tolerability to Faropenem 200 mg t.i.d: 91.4 %
Conclusion:
Since Faropenem is orally bioavailable and has been demonstrated to have strong activity against respiratory pathogens, it holds an excellent therapeutic promise. Overall, Faropenem is effective both clinically and bacteriologically, and was also well tolerated. A ten-day course of Faropenem 200 mg thrice daily regimen is a promising treatment of CAP in adults.6
Objective: To compare and examine the clinical effects of Faropenem sodium and Cefdinir for bacterial skin infection.
N = 62
Results: Faropenem 600 mg/day, Cefdinir 300 mg/day
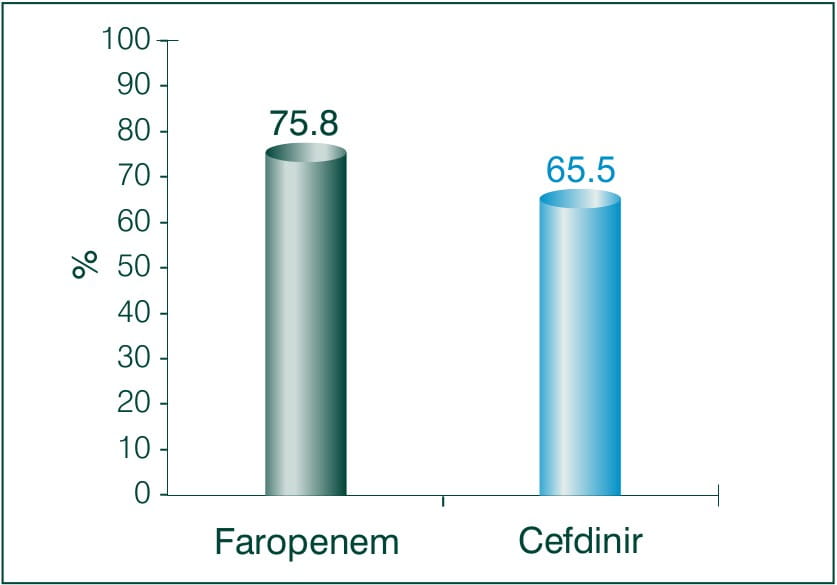
Conclusion: There was no significant difference between both the treatment groups, neither any adverse reaction occurred. Thus the study demonstrates the clinical efficacy and safety of Faropenem equivalent to Cefdinir in patients with deep skin tissue infections.7
Objective: To evaluate the clinical efficacy of Faropenem, in the field of obstetrics and gynaecology.
N = 165 ambulatory patients
Results: 3-7 days of Faropenem 600 mg/day
Clinical efficacy:
Overall efficacy rate to treat Obstetrics and Gynaecology: 94.5%
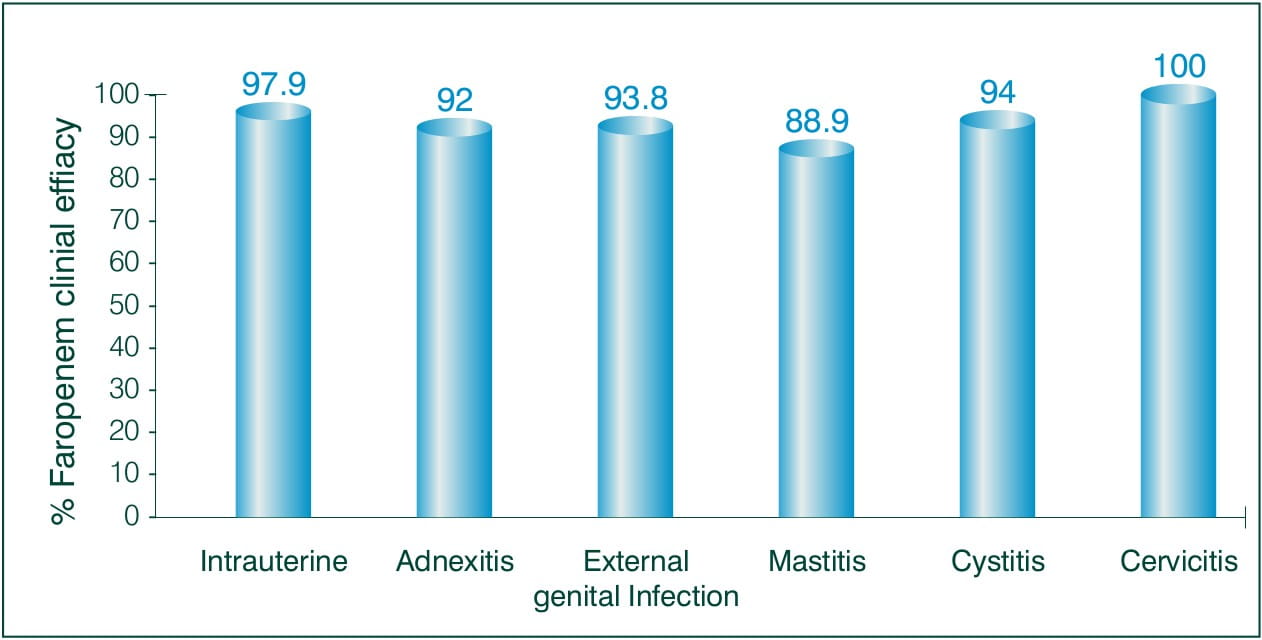
Conclusion: The results suggest that Faropenem is a highly safe and effective antibiotic for the treatment of obstetric or gynaecological infections of various kinds in an ambulatory setting.8
Objective: Faropenem 300 mg 3 times daily versus levofloxacin 100 mg 3 times daily in the treatment of urinary tract infections in patients with neurogenic bladder and/or benign prostatic hypertrophy.
N = 60 with significant bacteriuria and pyuria
Results:
Overall efficacy rate (excellent plus moderate) was achieved in 90.6% (29/32) of patients treated with Faropenem versus 82.1% (23/28) of those treated with Levofloxacin.
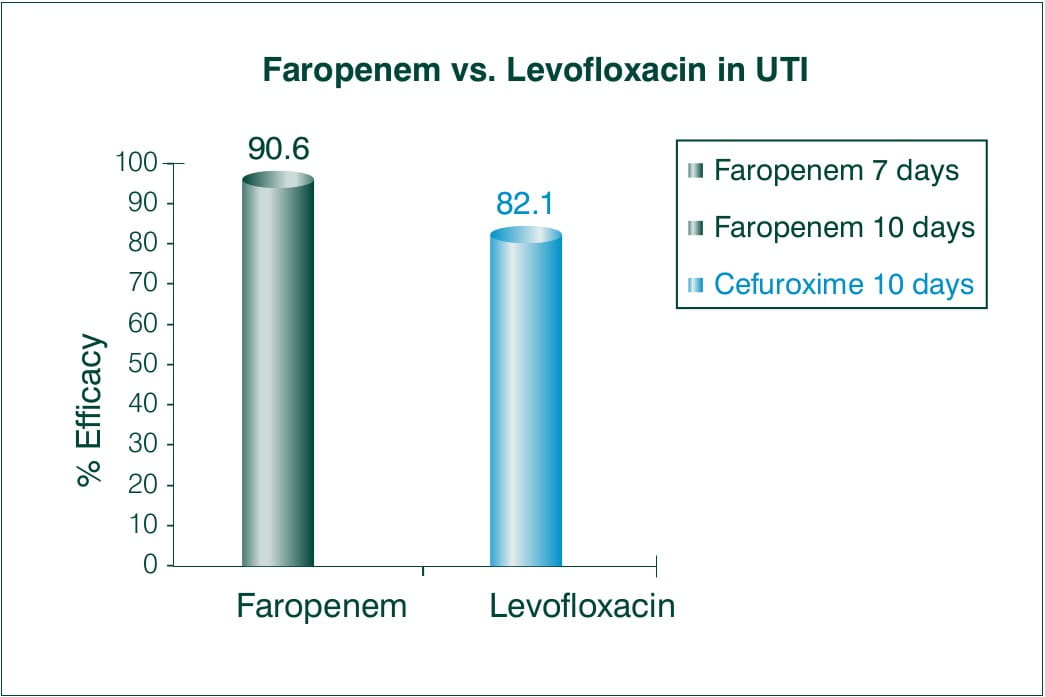
The ratios of eliminated bacteriuria and cleared pyuria were 71.9% and 56.3% of patients treated with Faropenem, and 64.3% and 75.0% of those treated with Levofloxacin.
Conclusion:
There was no significant difference between both the regimens used to treat urinary tract infection. In conclusion, Faropenem 300 mg 3 times daily is as effective as Levofloxacin 100 mg 3 times daily in patients with complicated urinary tract infection.9
Faropenem is contraindicated in patients with known hypersensitivity to any of the components of this product or to other drugs in the same class or in patients who have demonstrated anaphylactic reactions to beta-lactams.
Patients with past history of hypersensitivity to penicillin, cephem or carbapenem drugs.
Patients with family history of atophy.
Patients with renal insufficiency. The dosage should be reduced or the interval between doses should be increased.
Geriatric patients.
Patients with poor oral intake or poor general state (since there are cases that show symptoms of vitamin K deficiency proper monitoring should be done).
General
There is a fear of occurrence of anaphylactic shock; a thorough medical history should be taken.
Diarrhoea and loose bowel movements are frequently reported with faropenem. Incase it occurs, appropriate measures, such as discontinuation of faropenem etc, should be taken.
In case of geriatrics, since occurrence of diarrhoea and loose bowel movement results in deterioration of the general state, the patient must be directed to get immediate instructions from the doctor as soon as such symptoms appear and at the same time the use of faropenem should be stopped and appropriate measures should be taken.
Imipenem and cilastatin sodium combination: It has been reported that in animal studies (rat), the concentration of faropenem in blood increases. It is due to obstruction of metabolic fermentation by cilastatin.
Furosemide: It has been reported in animal studies (dog), that the kidney toxicity of faropenem increases.
Sodium valproate: It has been reported that due to joint usage with carbapenem drugs (meropenem, panipenem and imipenem-cilastatin sodium), the concentration of valproic acid in blood reduces, and there is recurrence of epileptic fits.
Safety regarding therapy during pregnancy has not been established. In pregnant women or expectant mothers, the medicine should be given only if the benefits of the treatment are greater than the risk involved.
Faropenem is excreted in human milk. In lactating women, faropenem should be given only if the benefit outweighs the risk.
Renal Impairment
In patients with renal impairment, it was found that the plasma concentration of the drug is increased and the half-life is extended.
Paediatrics
Safety regarding therapy in infants has not been established.
Geriatric Use
Half life of faropenem is prolonged in the elderly and this may be due to decline in kidney function and as a result there is high plasma concentration. In the elderly, start with a dose of 150 mg and monitor the patient for any undesirable effects. If diarrhoea and loose bowel movements appear, stop the medicine, monitor properly and take appropriate measures. There is a tendency of haemorrhage due to vitamin K deficiency in the elderly.
Faropenem is generally well tolerated. The most frequently reported adverse reactions are diarrhoea, abdominal pain, loose bowel movements, nausea and rash.
The following adverse reactions have been observed:
Shock, pseudoanaphylactic symptoms: feeling of discomfort, wheezing, breathing trouble, dizziness, feeling of need to evacuate bowel, ringing in the ear, sweating, flushing of whole body, vascular edema, low blood pressure.
Acute renal insufficiency
Serious colitis accompanied by pseudomembranous colitis: bloody stool, stomachache, frequent diarrhoea.
Mucocutaneous Ocular Syndrome (Steven-Johnson syndrome), Toxic Epidermal Necrosis (Lyell syndrome).
Interstitial Pneumonia: pyrexia, cough, breathing trouble, abnormalities in the chest x-ray. If these symptoms appear, appropriate measures such as adrenal cortical hormone should be administered.
Liver function disorder, jaundice: an increase in AST (SGOT), ALT (SGPT), ALP etc. Agranulocytosis.
Striated muscle softening: muscular pain, feeling of exhaustion, increase in CK (CPK), increase in myoglobin in blood and in urine, and subsequently, acute renal insufficiency.
Pulmonary infiltration with eosinophillia (PIE) syndrome: pyrexia, cough, breathing trouble, abnormalities in the chest x-ray and eosinophillia. If these symptoms appear, appropriate measures such as adrenal cortical hormone should be administered.
Hypersensitivity reactions: rash, pyrexia, redness, hives, red spots on skin, etc.
Abnormal laboratory findings: e.g. increases in liver function tests (ALT, AST, bilirubin, LDH etc.), eosinophillia, increase in BUN-Creatinine, changes in granulocytes and platelets.
Vitamin deficiency: Symptoms of vitamin K deficiency (low prothrombin, tendency of haemorrhage etc.), symptoms of vitamin B-group deficiency (inflammation of tongue, stomatitis, lack of appetite, neuritis etc.) might occur rarely.
Gastrointestinal disorders: Vomiting, stomachache, diarrhoea, lack of appetite, inflammation of corners of mouth and lips, gastritis, constipation, stomatitis.
Others: Burning sensation, headache, dizziness, drowsiness, edema, dryness of mouth and lips, change in nail colour, washed out feeling occurs rarely.
No specific information is available on the treatment of overdosage with faropenem. Intentional overdosing of faropenem is unlikely. In the event of an overdose, faropenem should be discontinued and general supportive treatment given until renal elimination takes place.
Store in a cool dry place. Protect from moisture.
Farobact 200 - Strip pack of 6 Tablets
1. IDrugs. 2000 dec; 3(12):1399-401.
2. J Antimicrob Chemother. 1997; 39(1):35-43.
3. Antimicrob Agents Chemother. 2002; 46(2):550-5.
4. JAC 2002, 49: 573-584
5. Expert Rev Anti Infect Ther. 2007; 5(2):185-98.
6. The Ind. Pract. 2007; 60(4): 225-231
7. Journal of Clinical Therapeutics & Medicines 2006, vol.22 (5): 443-456
8. Jpn J Antibiot. 1999; 52 (7): 504-10
9. Kansenshogaku Zasshi. 2002 nov; 76(11 ):928-38