SUPREME: Post Marketing Surveillance to Evaluate the Safety and Efficacy in Hypertension of the Combination of Amlodipine & Nebivolol (Amlopres-NB).
Study Title
Aim
To evaluate the safety and efficacy of the fixed-dose combination of amlodipine and nebivolol (Amlopres-NB) in hypertensives.
Patients
Hypertension (N=673)
Exclusion Criteria
- Hypersensitivity to either component
- Severe bradycardia
- Heart block greater than first degree
- Cardiogenic shock
- Decompensated cardiac failure
- Sick sinus syndrome (unless a permanent pacemaker is in place)
- Severe hepatic impairment (Child-Pugh >B)
Study Design
- Patients were prescribed one tablet of Amlopres-NB once daily
- Follow-up visits at week 2 and week 4
- SBP, DBP and effect on angina assessed at baseline, week 2 and week 4
- Pulse rate & clinical examination done at all occasions
Results
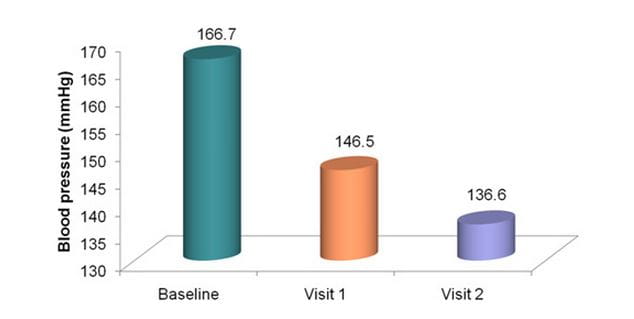
Significant reduction in SBP
P <0.0001 for visit 1 vs. baseline, visit 2 vs. visit 1 and visit 2 vs. baseline
N = 673 hypertensives from 136 clinics across India
Duration = 4 weeks
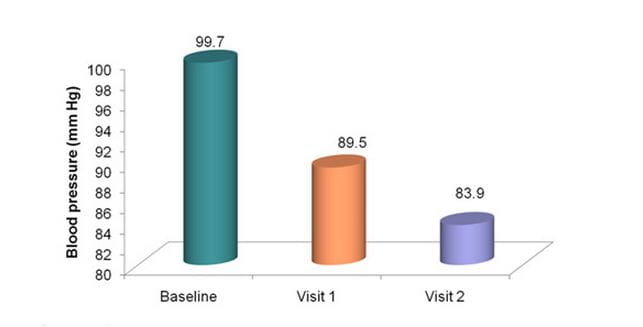
Significant reduction in DBP
Duration = 4 weeks
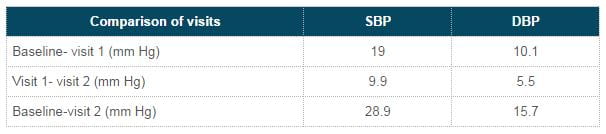
Change in BP
P<0.0001 for visit 1 vs. baseline, visit 2 vs. visit 1 and visit 2 vs. baseline
Effect on angina
No. of patients with angina
Frequency of anginal episodes
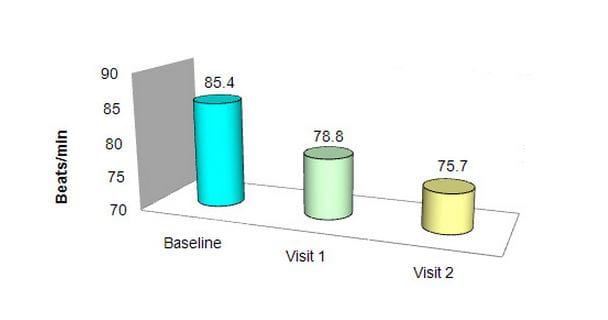
Effect on pulse rate
P<0.0001 for visit 1 vs. baseline, visit 2 vs. visit 1 and visit 2 vs. baseline
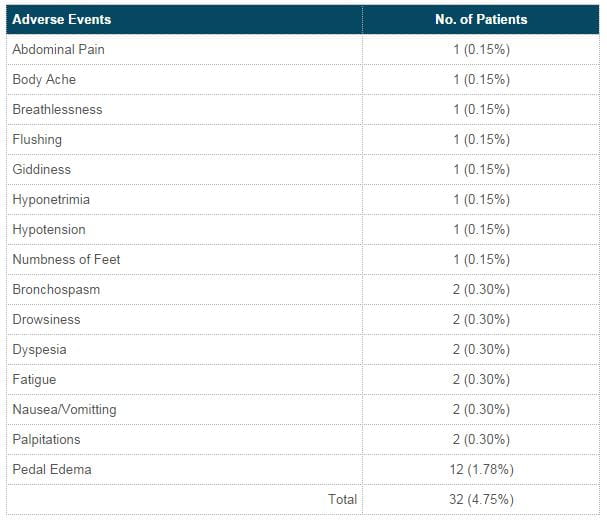
Adverse events (27 pts)
Mild nature (26) and moderate severity (6)
- Association
- Possible-7
- Probable-6
- Certain-4
- Conditional-1
- Unlikely-1
- 2 patients withdrawn due to pedal edema
Conclusion
Thus, Amlopres-NB is effective and well tolerated in Indian hypertensives.
Indian Pract. 2009; 62(9):565-70