Zoledronic Acid: An Effective, Safe and Convenient Alternative to Pamidronate for Reducing Long-Term Skeletal Morbidity in Patients with Advanced Multiple Myeloma or Breast Cancer
Introduction
Metastatic bone lesion, a common complication in patients with breast cancer or multiple myeloma (MM), substantially increases the long-term risk of developing skeletal complications. Although pamidronate significantly delays the onset and curbs the incidence of skeletal complications, its lengthy infusion time (2-4 hours every 3-4 weeks) limits its use. Zoledronic acid with a reduced infusion time (15 minutes) is superior to pamidronate for treating hypercalcemia of malignancy (HCM). It also has a proven efficacy in patients with bone metastases from solid tumors other than breast carcinoma. A 13-month comparable safety and efficacy profile of zoledronic acid vs. pamidronate has already been established.
Aim
To compare the long-term (25-month) safety and efficacy of zoledronic acid vs. pamidronate in patients with bone lesions secondary to advanced breast carcinoma or MM.
Patient Profile
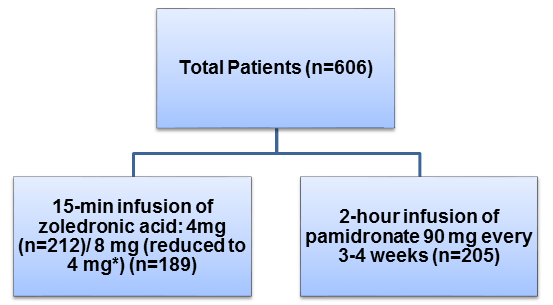
- Adult patients (n=606) with at least 1 osteolytic bone lesion secondary to Durie–Salmon stage III MM or at least 1 bone metastasis (osteolytic, osteoblastic, or mixed) secondary to stage IV breast carcinoma, Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, corrected serum calcium < 12 mg/dL, serum creatinine ≤ 3 mg/dL, and serum bilirubin ≤ 2.5 mg/dL
- All patients were on an ongoing antineoplastic therapy or received hormonal therapy at the time of randomization
- None of the patients had received bisphosphonates within 12 months of screening visit
Methods
Study Design
- Randomized, multicenter, stratified, double blind, double-dummy, parallel group, phase III trial
Treatment Strategy
- Patients were randomized to receive one of the following medication in combination with standard antineoplastic therapy for 24 weeks
*8 mg zoledronic acid reduced to 4 mg is referred to as; 8/4 mg zoledronic acid
- All patients were administered 500-mg calcium supplement and 400–500 International Units (IU) of vitamin D daily
Primary Outcome
- The proportion of patients with at least 1 skeletal-related event ([SRE]; pathologic fracture, spinal cord compression, radiation therapy, or surgery to bone)
Secondary Outcomes
- Time to first SRE, skeletal morbidity rate, and multiple-event analysis, overall survival, and ECOG performance status changes
- Hypercalcemia of malignancy was included as an SRE during secondary analyses
Results
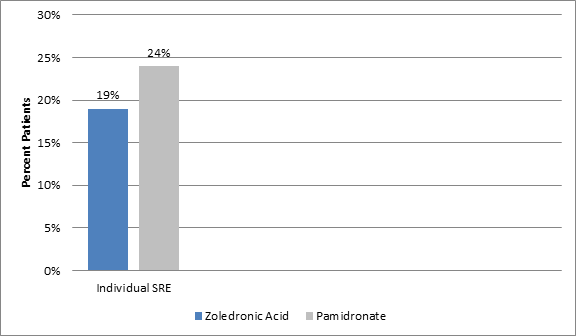
- The incidence of individual type of SRE was consistently lower in patients on zoledronic acid (4 mg) vs. pamidronate (p=0.037) (Figure 1)
- A 25% reduction in the mean annual incidence of skeletal complications (skeletal morbidity rate) was evident for patients treated with 4 mg zoledronic acid vs. those treated with pamidronate (1.04 events/year vs. 1.39 events/year; p=0.084). The reductions were more prominent in the hormonal-therapy treated breast cancer patients (0.83 SREs/year vs. 1.37 SREs/year, p=0.039)
- The 4 mg zoledronic acid treatment was associated with a 16% greater reduction in the risk of developing a skeletal complication as compared to 90 mg pamidronate treatment (risk ratio [RR]; 0.841). The hormonal-therapy treated breast cancer patients receiving 4 mg zoledronic acid particularly demonstrated a 30% additional risk reduction for developing skeletal complication as compared to those treated with pamidronate (RR; 0.693, p=0.009)
- A significant 20% lower skeletal morbidity was evident in breast cancer patients treated with 4 mg zoledronic acid vs. pamidronate (RR; 0.799; p=0.025)
- Fewer patients treated with 8/4 mg zoledronic acid required surgery (4% vs. 6%; p=0.049) or developed HCM (1% vs. 3%; p=0.025) as compared to those treated with pamidronate
- Although the median time to first skeletal complication was similar in patients treated with 8/4 mg zoledronic acid, the 8/4 mg zoledronic acid patients had a 28% lower mean annual incidence of skeletal events as compared to the pamidronate patients (1.00 vs. 1.39 events/year; p=0.227) along with a trend towards risk reduction for developing skeletal complications including HCM (RR; 0.854, p=0.052)
Safety Profile
- The incidence of adverse events (AEs) was similar in the treatment groups and both the drugs were well tolerated
- Bone pain, fatigue and nausea were the most common AEs. Most of the adverse events were of mild to moderate severity.
- The long-term renal safety profile for 4 mg zoledronic acid vs. pamidronate was not significantly different. But, 8/4 mg zoledronic acid patients had a two-times greater risk of decreased renal function vs. pamidronate (RR; 2.187)
Conclusions
- Zoledronic acid demonstrated a long-term (25 months) safety and efficacy comparable to that of pamidronate in patients with MM and breast carcinoma
- The long-term treatment with zoledronic acid was not associated with increased risk of renal impairment
- The ease of administration for zoledronic acid (15-minutes infusion) as compared to tedious infusion of pamidronate (2-4 hours) was an added convenience benefit that might positively impact the quality of patients’ life and reduce the utilization of health-care resources
Cancer 2003; 98: 1735–44