Introduction
The Understanding Potential Long-Term Impacts on Function with Tiotropium (UPLIFT) trial was designed to assess the benefits of tiotropium in reducing the rate of decline in FEV1 in patients with chronic obstructive pulmonary disease (COPD). Tiotropium is a once-daily, inhaled anticholinergic drug that delivers long-lasting 24-hour improvements in airflow and hyperinflation in patients with COPD.
Aim
The trial focused on the long-term effects of tiotropium therapy on the clinically important end points of health-related quality of life (HRQoL), exacerbations, related hospitalizations, and mortality.
Patients Profile
- N=5993
- Age of ≥ 40 years
- Smoking history of at least 10 pack-years
- Post-bronchodilator FEV1 of ≤ 70% of the predicted value
- FEV1 / FVC of ≤ 70% or less
- Exclusion criteria: History of asthma, a COPD exacerbation or respiratory infection within 4 weeks before screening, a history of pulmonary resection, use of supplemental oxygen for more than 12 hours per day, and the presence of a coexisting illness that could preclude participation in the study or interfere with the study results
Method
Study Design
- A 4-year, randomized, double blind, placebo-controlled, parallel-group trial involving patients with moderate-to-very-severe COPD
- The patients were from 490 investigational centers set in 37 countries
Dosing
- Patients were divided into 2 groups:
- Those who received 18 ?g of tiotropium; or
- Those who received a matching placebo once daily
- The patients received doses via HandiHaler inhalation device
- All respiratory medications, except other inhaled anticholinergic drugs, were permitted during the trial
- At the end of the study, all patients were given 40 ?g of ipratropium (two inhaler actuations) four times daily and to return for a final assessment after 30 days
End-point
- Primary end points: yearly rate of decline in the mean FEV1:
- Before the use of a study drug and short-acting bronchodilators in the morning (prebronchodilator) and
- After the use of a study drug (postbronchodilator) from day 30 (steady state) until completion of double-blind treatment
- Secondary end points:
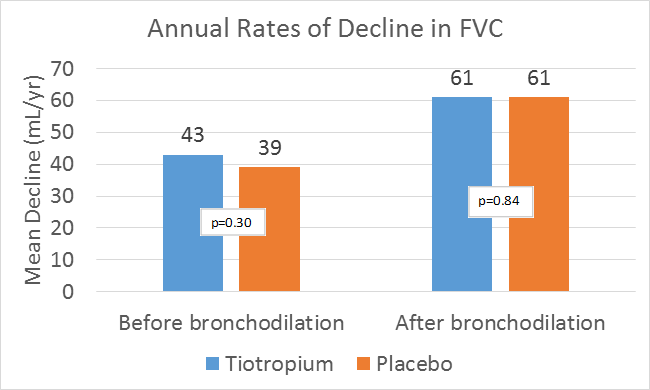
- Rate of decline in the mean forced vital capacity (FVC) and slow vital capacity (SVC)
- Health-related quality of life, as measured by the total score on St. George’s Respiratory Questionnaire (SGRQ)
- Exacerbations of COPD and related hospitalizations. Rate of death from any cause and from lower respiratory conditions
Results
Rate of Decline in Lung Function
- The mean absolute improvements in FEV1 in the tiotropium group were maintained throughout the trial (ranging from 87 to 103 mL before bronchodilation and from 47 to 65 mL after bronchodilation), as compared with the placebo group (P<0.001).
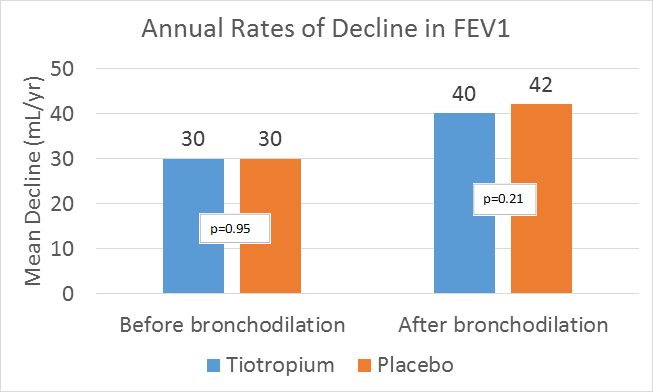
- After day 30, the differences between the two study groups in the rate of decline in the mean FEV1 and FVC before and after bronchodilation remained non-significant.
Health-Related Quality of Life
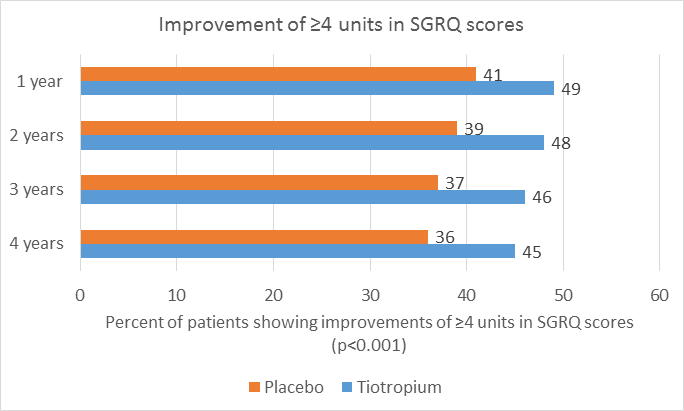
- The mean absolute total score on the SGRQ was improved (i.e. lower) in the tiotropium group, at each time point throughout the 4-year period (ranging from 2.3 to 3.3 units, P<0.001) as compared with the placebo group.
Exacerbations
- Exacerbations were defined as an increase in or the new onset of more than one respiratory symptom (cough, sputum, sputum purulence, wheezing, or dyspnea) lasting 3 days or more and requiring treatment with an antibiotic or a systemic corticosteroid.
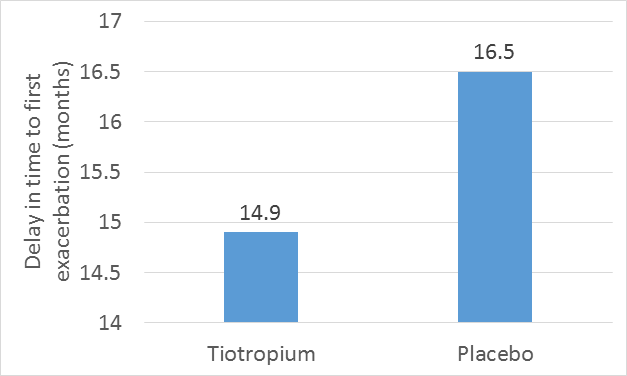
- Tiotropium was found to be associated with a reduction in the mean number of exacerbations of 14% (P<0.001) and a significant delay in the time to the first hospitalization for an exacerbation.
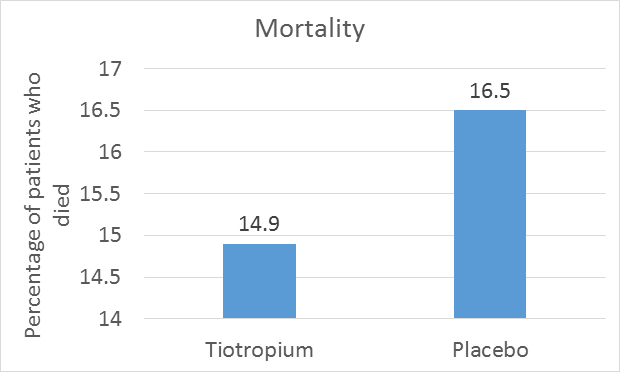
Mortality
- During a period of 4 years plus 30 days (1470 days) of the study, a total of 941 patients died: 14.9% in the tiotropium group and 16.5% in the placebo group.
Safety
- Tiotropium showed reduced respiratory morbidity (including a decreased risk of respiratory failure) and reduced cardiac morbidity as compared to the placebo
|
Variables |
Tiotropium |
Placebo |
|
Adverse events reported |
92.6% |
92.3% |
|
Serious adverse events |
51.6% |
50.2% |
|
Fatal events |
12.8% |
13.7% |
|
Adverse Event
|
Tiotropium (N = 2986) |
Placebo (N = 3006) |
Relative Risk for Tiotropium vs. Placebo |
|
Cardiac |
3.56 |
4.21 |
0.84 † |
|
Angina |
0.51 |
0.36 |
1.44 |
|
Atrial fibrillation |
0.74 |
0.77 |
0.95 |
|
Cardiac failure |
0.61 |
0.48 |
1.25 |
|
Congestive heart failure |
0.29 |
0.48 |
0.59 † |
|
Coronary artery disease |
0.21 |
0.37 |
0.58 |
|
Myocardial infarction |
0.69 |
0.97 |
0.71† |
† P<0.05
- The most frequent adverse events encountered were due to lower respiratory causes, including COPD exacerbations, pneumonia and dyspnea, myocardial infarction and stroke development.
- Other adverse events consistent with the known safety profile of tiotropium, like dry mouth and constipation, were also observed
Conclusions
Tiotropium was shown to improve lung function, quality of life, and exacerbations during a 4-year period despite the non-significant reduction in rate of decline in FEV1; in patients with COPD.
N Engl J Med. 2008; 359(15):1543-54