Treatment with Macitentan Reduces Morbidity and Mortality in Patients with Pulmonary Arterial Hypertension ? SERAPHIN Study
Introduction
Previous trials with endothelin-receptor antagonists, phosphodiesterase type 5 inhibitors and prostacyclin and its analogues have shown improvements in exercise capacity in patients with pulmonary arterial hypertension (PAH). However, new treatments need to be evaluated using morbidity and mortality as primary endpoint as per the current guidelines. The structure of bosentan was modified to develop macitentan, a dual endothelin-receptor antagonist, to increase efficacy and safety.
Aim
The Study with Endothelin Receptor Antagonist in PAH to Improve Clinical Outcome (SERAPHIN) was conducted to assess whether morbidity and mortality events reduces with long term treatment with macitentan in PAH patients.
Methods
Study design
- Multicenter, double-blind, randomized, placebo-controlled, event-driven phase 3 trial
- Patients aged 12 years or more, with confirmed PAH and with 6-minute walk distance (6-MWD) of 50 m or more and class II, III or IV according to WHO functional classification were selected
- Sixty-one % of patients were concomitantly receiving oral phosphodiesterase inhibitors or inhaled therapy for PAH
- The cohort was randomized into 3 groups within 28 days of screening to receive placebo once daily, macitentan 3 mg once daily and macitentan 10 mg once daily
- The cohort was assessed for clinical and laboratory parameters at screening, at months 3 and 6 and every 6 months up to the end of treatment
Endpoints
- Time from initiation of the treatment to first occurrence of a composite endpoint of death, atrial septostomy, lung transplantation, initiation of treatment with intravenous or subcutaneous prostanoids or worsening of PAH
Results
- The overall cohort comprised of 742 patients
- 250 received placebo, 250 received macitentan 3 mg and 242 received macitentan 10 mg
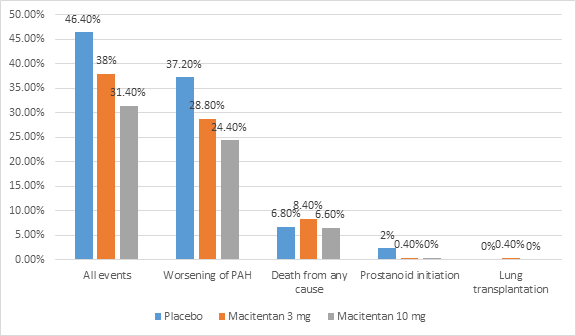
- Occurrence of primary end point event was reported in 46.4% of placebo group, 38% of macitentan 3 mg group and 31.4% of macitentan 10 mg group
- The risk of primary endpoint event was higher in placebo as compared to macitentan 3 mg group (HR 0.70; p=0.01) and macitentan 10 mg (HR 0.55; p<0.001)
- The most frequent primary end point event was worsening of PAH
- The proportion of patients with occurrence of primary endpoint event is shown in figure 1.
- The composite endpoint of death due to PAH or PAH-associated hospitalization occurred in 33.6%, 26% and 20.7% in placebo, macitentan 3 mg and macitentan 10 mg groups respectively.
- Placebo group was at a higher risk of death or hospitalization as compared to macitentan 3 mg group (HR 0.67; p=0.01) and macitentan 10 mg group (HR 0.50; p<0.001).
- 6-MWD decreased by a mean of 9.4 m in placebo group
- 6-MWD increased by a mean of 7.4 m and 12.5 m in macitentan 3 mg and 10 mg groups (p=0.01 and p=0.008) respectively at month 6
- 13%, 20% and 22% demonstrated improvement in WHO functional class at month 6 in placebo, macitentan 3 mg and 10 mg groups respectively
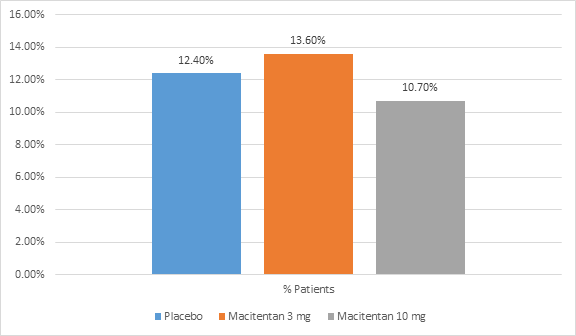
- The proportion of patients who discontinued the study drug due to adverse events in all the groups is shown in figure 2.
- More frequent adverse events in the macitentan groups were headache, nasopharyngitis and anemia
Conclusion
- Treatment with macitentan resulted in significant reduction in morbidity and mortality in patients with pulmonary arterial hypertension.
- The risk of clinical failure and hospitalization was reduced by macitentan in patients with PAH
N Engl J Med. 2013 Aug 29;369(9):809-18. Doi: 10.1056/NEJMoa1213917.