Study 102-103: Tenofovir in Chronic Hepatitis B
Chronic hepatitis B is a major public health problem affecting up to 400 million people globally. Complications of chronic hepatitis B, including liver failure and hepatocellular carcinoma, result in 1.2 million deaths per year, making it the tenth leading cause of mortality worldwide; hence, it can aptly be termed 'the silent killer'.
Tenofovir disoproxil fumarate, a nucleotide reverse transcriptase inhibitor, has potent activity against the hepatitis B virus and received approval in 2008 from the U.S. Federal Drug Administration and the European Commission (EMEA), and is now approved in India as a treatment for chronic hepatitis B in adults. Clinical Practice Guidelines recommend the use of tenofovir disoproxil fumarate as first-line monotherapy in patients with chronic hepatitis B.
Tenofovir disoproxil fumarate fulfills the criteria of an ideal anti-hepatitis B virus drug in all aspects. Its formidable antiviral efficacy spans a wide spectrum of patient subgroups and can tackle both the wild-type and precore -core mutants in treatment-naive as well as treatment-experienced patients. Till date, resistance to tenofovir disoproxil fumarate has not been detected in patients of chronic hepatitis B and it has been safe and well tolerated.
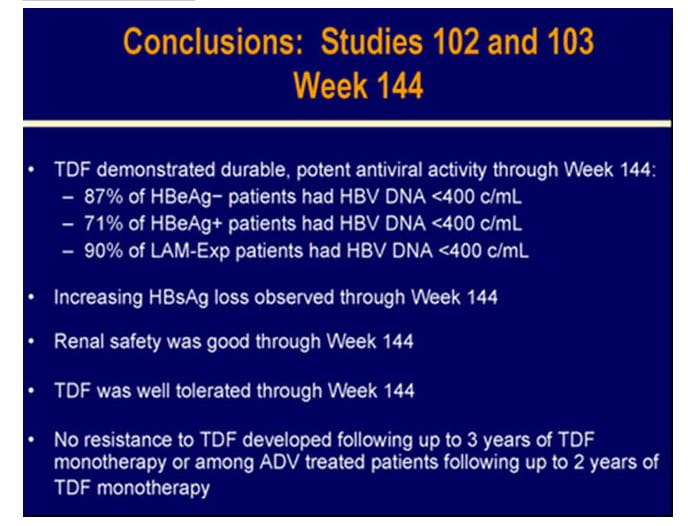
TENVIR is indicated for the treatment of chronic hepatitis B in adults with compensated liver disease. Two important landmark clinical trials STUDY 102 and STUDY 103 in HBeAg negative and HBeAg positive patients respectively (both ongoing trials scheduled for 8 years) have periodically assessed efficacy and safety of tenofovir in chronic hepatitis B patients.
To assess the 96-week efficacy and safety of tenofovir and of a switch to tenofovir after 48 weeks of adefovir in HBeAg-negative (N=375) and HBeAg-positive patients (N=266) with CHB mono-infection.
- Patients with HBe Ag-negative or HBeAg-positive CHB mono-infection were randomized 2:1 to receive either tenofovir DF (TDF) 300 mg or adefovir (ADV) 10 mg once daily and it was a double blind study for the first 48 weeks.
- In study 102, for the 48-week period, N=250 patients were on tenofovir DF and N=125 patients on adefovir.
- Similarly in study 103, N=176 patients were on tenofovir DF (TDF) 300 mg and N =90 were on adefovir 10 mg once daily for the first 48 weeks.
- After 48 weeks, all patients (both tenofovir and adefovir groups) were switched to open-label tenofovir DF with a liver biopsy at week 48, for an additional 7 years in both study 102 and study 103.
- Option to initiate combination of emtricitabine plus tenofovir DF treatment at or after week 72 for confirmed HBV >69 IU/mL.
- Analyses were conducted at 48, 72 and 96 weeks.
- Liver biopsies were conducted at recruitment and at week 48.
Figure.1 Study 102 and Study 103 of Tenofovir DF in HBeAg-Negative and HBeAg-Positive Patients, Respectively
TDF = Tenofovir DF; ADV = Adefovir
Patients of study 102 (HBeAg negative)
# Patients of study 103 (HBeAg positive)
- 18-69 years of age.
- CHB mono-infection.
- HBeAg-Negative disease or HBeAg-Positive disease.
- HBV DNA >106 copies/mL (HBeAg-Positive) or HBV DNA >105 copies/mL (HBeAg-Negative) and ALT 2-10 x ULN (HBeAg-Positive) or 1-10 x ULN (HBeAg-Negative) but no more than 10 times ULN in either.
- Naive- or lamivudine-experienced.
- Knodell necroinflammatory score ≥3.
Subjects were treatment-naive, defined as less than 12 weeks of treatment with any nucleoside or with the nucleotides tenofovir DF or adefovir dipivoxil, or treatment with lamivudine or emtricitabine of any duration. However, the protocol was amended to allow up to 120 subjects to enroll with more than 12 weeks of prior treatment with lamivudine or emtricitabine. Previous treatment with a nucleoside, nucleotide, or interferon (pegylated or not) must have ended 6 months prior to the required pretreatment liver biopsy.
- Hepatitis delta virus (HDV), hepatitis C virus (HCV), HIV/HBV co-infection
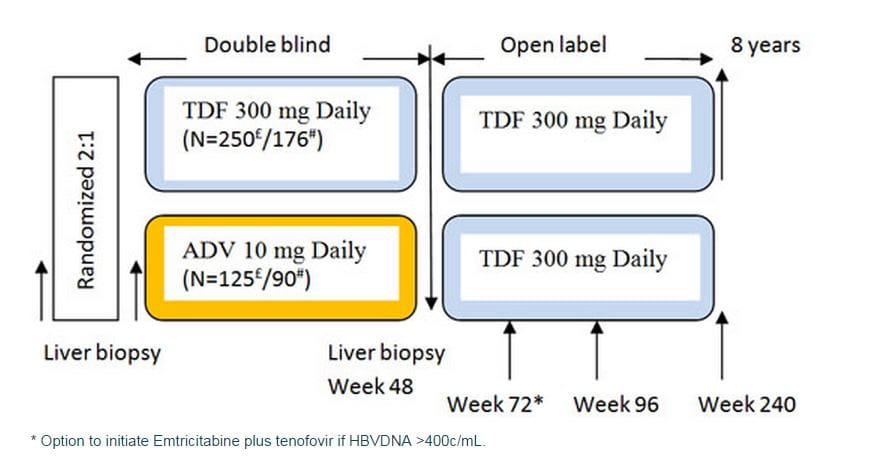
- Baseline demographics and disease characteristics were well balanced between the treatment arms (Table).
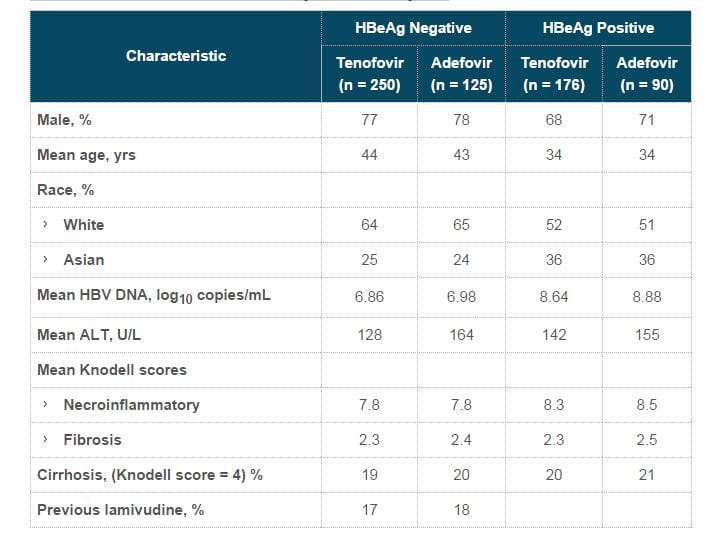
- Virologic response was defined as HBV DNA <400 copies/mL or <69 IU/mL . Three subjects (all in the TDF-TDF group) were switched to open-label emtricitabine/tenofovir during the open-label period due to confirmed viraemia. One of these 3 subjects had achieved complete viral suppression by Week 96.
- At the end of 96 weeks, both the continuous tenofovir DF group and the adefovir to tenofovir DF switch group showed undetectable HBV DNA levels in 90% and 89% of subjects, respectively.
Figure.2 Virological response with HBV DNA Undetectability with tenofovir DF in Study 102 at varied time intervals

TDF = Tenofovir DF; ADV = Adefovir
- Sixteen subjects in the TDF-TDF group and 13 subjects in the ADV-TDF group switched to open-label emtricitabine/tenofovir during the open-label period due to confirmed viraemia. Twenty-three of these subjects never achieved viral suppression <400 copies/mL at any time during the study up to Week 96.
- At the end of 96 weeks, 78% of patients in both the continuous tenofovir DF group and the adefovir to tenofovir DF switch group showed undetectable HBV DNA levels.
Figure.3 Virologic response and HBV DNA Undetectability with tenofovir DF in Study 103 at varied time intervals.
TDF = Tenofovir DF; ADV = Adefovir
- At week 96, HBeAg loss was seen in 30% of patients in the continuous tenofovir DF group and 28% in the switch arm (adefovir to tenofovir DF).
- HBeAg seroconversion was seen in 26% of patients in the continuous tenofovir DF group and 24% in the switch arm (adefovir to tenofovir DF).
- HBsAg loss was 6% in both groups.
Figure.4 Serological response at week 96 in Study 103
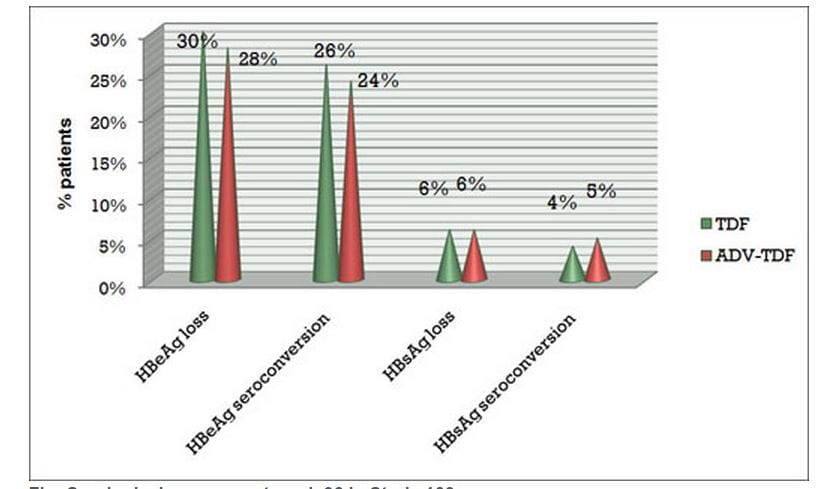
TDF = Tenofovir DF; ADV = Adefovir
- Mean ALT values were similar at week 96 for adefovir-tenofovir DF and tenofovir DF-tenofovir DF patients, with an overall mean ALT value of 37 U/mL. Biochemical response at 48 and 72 weeks.
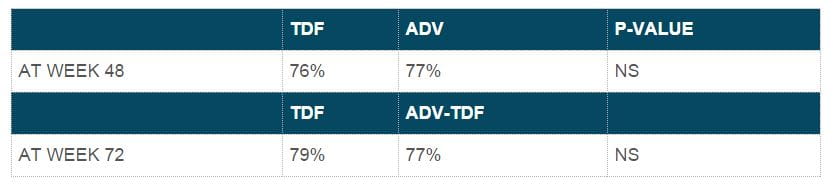
- At week 96, the overall mean ALT value was 36 U/L, with no significant differences between the groups. Biochemical response at 48 and 72 weeks.
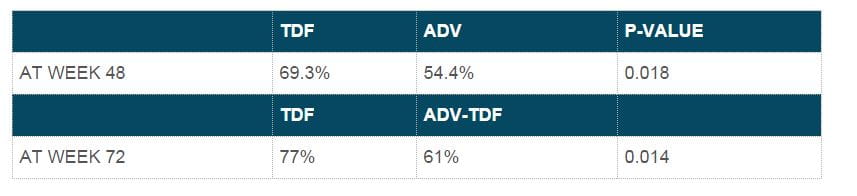
- At week 48, both drugs produced similar histological responses in 72.4% of subjects in the tenofovir DF group and 68.8% in the adefovir group.
- At week 48, both drugs produced similar histological responses in 74.4% in the tenofovir DF group and 68% in the adefovir group.
- No tenofovir DF-resistance mutations were found through 96 weeks of tenofovir DF monotherapy.
HBeAg-Negative and HBeAg-Positive Patients
- In both groups, tenofovir DF produced potent, continuous viral suppression through 96 weeks.
- Tenofovir DF was well tolerated in HBeAg-positive and HBeAg-negative atients.
- Patients switching to tenofovir DF after 48 weeks of adefovir treatment had significant additional viral suppression similar to those treated with tenofovir DF for 96 weeks.
1. Marcellin P, Buti M, Krastev Z, et al. Two year tenofovir disoproxil fumarate (TDF) treatment and adefovir dipivoxil (ADV) switch data in HBeAg-negative patients with chronic hepatitis B (study 102), preliminary analysis. Program and abstracts of the 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31 - November 4, 2008; San Francisco, California. Abstract 146.
2. Marcellin P, Buti M, Krastev Z, et al. A randomized, double-blind, comparison of tenofovir DF (TDF) versus adefovir dipivoxil (ADV) for the treatment of HBeAg-negative chronic hepatitis B (CHB): study GS-US-174-0102. Program and abstracts of the 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2-6, 2007; Boston, Massachusetts. Abstract LB2
3. Heathcote E, Gane EJ, deMan RA, et al. Two year tenofovir disoproxil fumarate (TDF) treatment and adefovir dipivoxil (ADV) switch data in HBeAg-positive patients with chronic hepatitis B (Study 103), preliminary analysis. Program and abstracts of the 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31 - November 4, 2008; San Francisco, California. Abstract 158.
4. Heathcote E, Gane E, DeMan R, et al. A randomized, double-blind, comparison of tenofovir DF (TDF) versus adefovir dipivoxil (ADV) for the treatment of HBeAg positive chronic hepatitis B (CHB): study GS-US-174-0103. Program and abstracts of the 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2-6, 2007; Boston, Massachusetts. Abstract LB6.
In study 102, patients with HBeAg-negative, CHB mono-infection were randomized 2:1 to double-blind, once daily TDF 300 mg (N=250) or adefovir dipivoxil 10 mg (ADV) (N=125) in the phase 3 study 102. After 48 weeks, patients with a week (WK) 48 biopsy were switched to open-label (OL) TDF for up to an additional 7 years with the option to add emtricitabine (FTC) to TDF (as a fixed dose combination tablet FTC/TDF) at or after WK72 for confirmed HBV DNA ? 400 copies (c)/mL (69 IU/mL).
HBV DNA was measured using the Roche COBAS TaqMan assay (LLQ=169c/mL=29 IU/mL). Patrick Marcellin et al. presented preliminary WK 144 (Year 3) data from this ongoing trial. Out of the 375 patients, 328 patients completed WK144.
Virologic Response
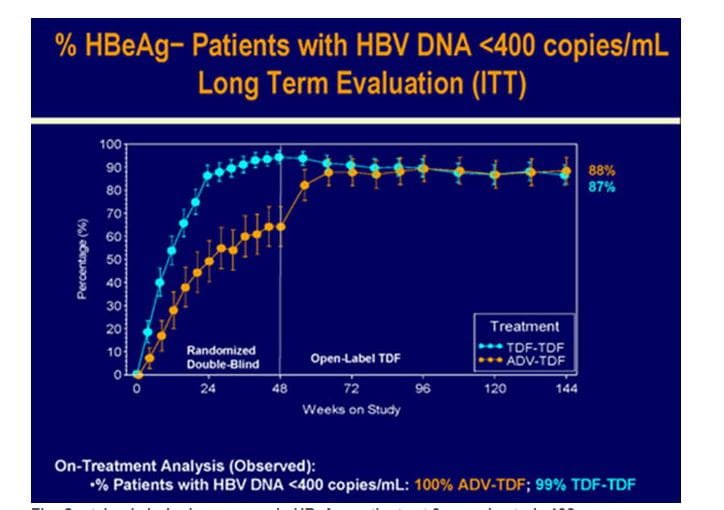
- 88% of the patients had HBV DNA <400 c/mL with no differences between the original randomized treatment groups. (Long Term Evaluation analysis :intent to treat; ITT),
- 99.1% of all patients on TDF at year 3 had HBV DNA <400 c/mL(on-treatment analysis )
- Three patients who initiated OL FTC/TDF at or after WK72 had HBV DNA ?400 c/mL at WK144 and one additional patient discontinued TDF during year 3 with HBV DNA ≥400 c/mL at the time of discontinuation.
Figure.5 Sustained virologic response in HBeAg- patients at 3 years in study 102
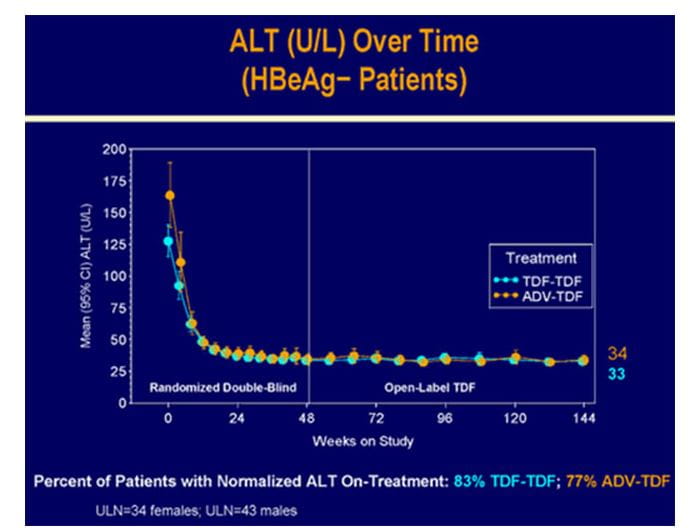
Biochemical Response
- The overall mean ALT was 38.5 U/L.
Figure.6 Biochemical response in HBeAg- patients at 3 years in study 102
Resistance
No mutations associated with TDF resistance were detected through year 3.
Safety
- Excellent renal safety of tenofovir was seen in year 3.Creatinine clearance remained stable throughout and no patient had a 0.5 mg/dL increase in creatinine or a decrease in creatinine clearance <50 mL/min.
- Only seven patients discontinued treatment during year 3; none discontinued therapy due to an AE.
- Two deaths occurred during year 3; none were considered related to study drug.
Hence TDF was well tolerated and produced potent, continuous viral suppression and no mutations associated with TDF resistance were detected through 3 years of TDF treatment.
Reference
1. Patrick Marcellin et al. Abstract 481, AASLD 2009 .Data presented at “The Liver Meeting” -Annual Meeting of AASLD 2009 - 144 weeks of study 102.
Treatment of patients with HBeAg+ CHB over two years in study 103 is associated with HBV DNA suppression in 78% of patients and HBsAg loss in 6% of patients (intent-to-treat analysis; ITT). The present analysis by E. Jenny Heathcote, et al. extends the observation of this treated cohort to 3 years (WK144). Patients with HBeAg+ CHB mono-infection were randomized 2:1 to once daily, double-blind TDF 300 mg (N=176) or adefovir dipivoxil 10 mg (ADV) (N=90) for the first 48 weeks.After 48 weeks, patients with a week (WK) 48 biopsy were switched to open-label (OL) TDF for up to an additional 7 years with the option to add emtricitabine (FTC)+TDF (as a fixed dose combination tablet FTC/TDF) at or after WK72 for confirmed HBV DNA ?400 c/mL (69 IU/mL). HBV DNA was measured using the Roche COBAS TaqMan assay (LLQ=169 c/mL=29 IU/mL). Among the 266 patients participating in the study, 214 patients completed WK144.
Virologic Response
- 72% of patients had HBV DNA <400 c/mL with no significant difference between the original treatment arms.( Long Term Evaluation analysis :intent to treat; ITT)
- 95% of the patients in the TDF for three years group and 91% of ADV to TDF switch group had HBV DNA <400 c/mL at WK144 (on-treatment analysis).
- 14 patients had HBV DNA ≥400 c/mL at WK144 and an additional 3 patients had HBV DNA ≥400 c/mL at their last available time point prior to TDF discontinuation during year 3.
- 31 patients had added OL FTC to TDF between WK72 and WK144 for HBV DNA ≥400 c/mL, 17 of whom had HBV DNA <400 c/mL at WK144.
Figure.7 Sustained virologic response in HBeAg+ patients at 3 years in study 103

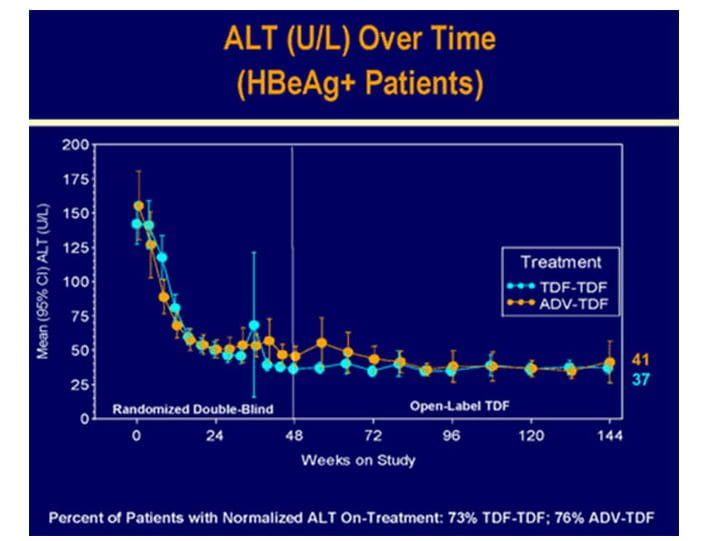
Biochemical Response
- The overall mean ALT was 38.5 U/L.
Figure.8 Biochemical response in HBeAg+ patients at 3 years in study 103
Serologic Response
- HBeAg loss and seroconversion was observed in 34% and 26% of patients, respectively (on-treatment analysis).Cumulatively, HBsAg loss and HBsAg seroconversion were observed in 8% and 5% of patients. Patients with HBsAg loss were predominantly genotype A and D.
Figure.9 Serological response in HBeAg+ patients at 3 years in study 103
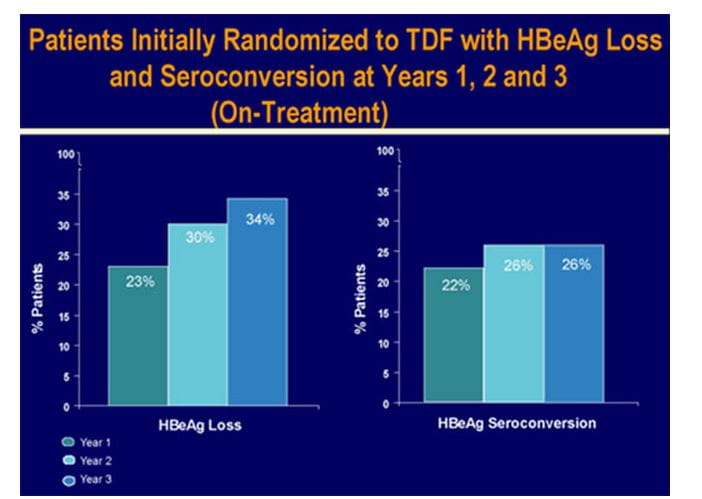
Figure.10 Cumulative probability of HBsAg loss with tenofovir treatment over 3 years

Resistance
- No mutations associated with TDF resistance were detected through year 3.
Safety
- 14 patients discontinued treatment between WK96 and 144, none discontinued due to an AE.
- There were no deaths during the study and no patient experienced an SAE on treatment considered related to TDF during year 3.
- One patient with a 0.5 mg/dL increase in creatinine during year 2 maintained this increase during year 3.Overall renal safety remained good.
Figure.11 Excellent renal safety of tenofovir over 3 years in HBV patients

Hence TDF was well tolerated and produced potent, continuous viral suppression and increasing serologic loss through 3 years of treatment in HBeAg positive Patients.
Reference
1. E. Jenny Heathcote et al. Abstract 483.Data presented at “The Liver Meeting” -Annual Meeting of AASLD 2009 -144 weeks of study 103.
2. http://www.natap.org/2010/APASL/APASL_11.htm accessed on July 30, 2010.