SMART-DATE Trial: Long-Term DAPT-The Standard of Care for ACS Patients Undergoing PCI with DES
7 Jun, 19
Background
Currently, dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor is recommended for at least 12 months after percutaneous coronary intervention (PCI) with drug-eluting stents (DES) in patients with acute coronary syndrome (ACS). Nevertheless, the data related to the optimal duration of DAPT following in ACS patients undergoing PCI with DES is not only limited but also controversial.
Aim
The aim of SMART-DATE (Safety of 6-month Duration of Dual Antiplatelet Therapy After Acute Coronary Syndromes) trial was to determine whether 6-month duration of DAPT would be non-inferior to the conventional 12-month or longer duration of DAPT (18 months) in ACS patients undergoing PCI
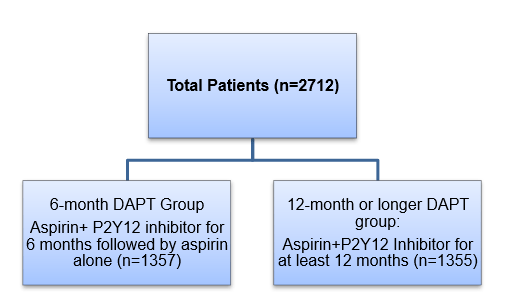
Patient Profile
- Patients with unstable angina, non-ST-segment elevation myocardial infarction (NSTEMI), or ST-segment elevation myocardial infarction (STEMI), who underwent PCI
- Patients had at least one lesion in a native coronary vessel with reference diameter of 2·25–4·25 mm and stenosis of more than 50% by visual estimation amenable for PCI with stents
Methods
Study Design
- Randomized, multicenter (conducted at 31 centers in South Korea), prospective open-label, non-inferiority trial
Treatment Strategy
- Patients were randomized as follows at the time of index procedure:
- All patients were treated with 300 mg of aspirin orally and clopidogrel loading dose (300 mg/ 600 mg) orally at least 12 h before PCI, unless they had previously received these antiplatelet medications.
- After the procedure, aspirin (100 mg orally once daily) was used indefinitely with clopidogrel (75 mg orally once daily) maintained as per the randomization scheme (6 months vs. 12 months or longer).
- Clopidogrel was used in 79·7% (n=1082) patients in the 6-month DAPT group and in 81·8% (n=1109) patients in the 12-month or longer DAPT group.
- Ticagrelor (180 mg followed by 90 mg twice daily, orally) and prasugrel (60 mg followed by 10 mg daily, orally) were used instead of clopidogrel in many cases once these medications were available in South Korea.
Endpoints
Primary Endpoint
- A composite of all-cause death, myocardial infarction (MI), or stroke at 18 months after the index procedure in the intention-to-treat population
Secondary Endpoints
- The individual components of the primary endpoint
- Definite or probable stent thrombosis (as defined by the Academic Research Consortium)
- Bleeding Academic Research Consortium (BARC) type 2–5 bleeding at 18 months after the index procedure
Follow-up
- 18 months
Results
- The median DAPT duration in the 6-month DAPT group was 6.1 months and that in the 12-month or longer group was 17.7 months.
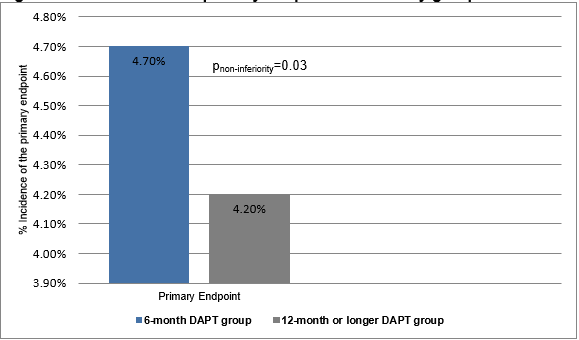
- The primary endpoint occurred in more number of patients in the 6-month DAPT group compared to those in the 12-month or longer DAPT group (cumulative event rate (4·7% vs. 4·2%; absolute risk difference 0·5%; upper limit of one-sided 95% CI 1·8%; pnon-inferiority=0·03 with a predefined non-inferiority margin of 2·0%) (Figure 1).
Figure 1: The incidence of primary endpoint in the study groups
- The incidence of all-cause mortality (2·6% vs. 2·9%; hazard ratio [HR] 0·90 [95% CI 0·57–1·42]; p=0·90) or stroke (0·8% vs. 0·9%, HR; 0·92 [0·41–2·08], p=0·84) did not differ significantly between the 6-month and the 12-month or longer DAPT groups.
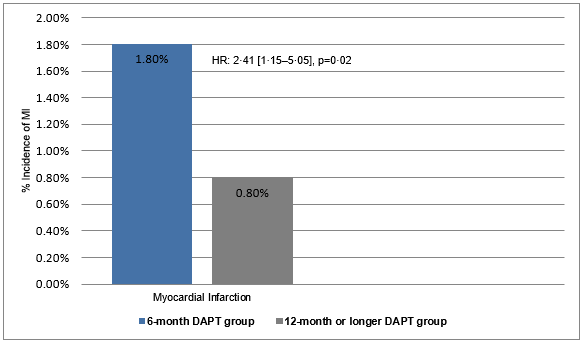
- Myocardial infarction (non-target vessel MI and target vessel MI) was more frequent in the 6-month DAPT group than in the 12-month or longer DAPT group (1·8% vs. 0·8%, HR; 2·41 [1·15–5·05], p=0·02) (Figure 2).
Figure 2: The incidence of myocardial infarction in the study groups
- The risk of stent thrombosis did not differ significantly between the 6-month DAPT group and the 12-month or longer DAPT group (1.1% vs. 0.7%; p=0·32). Also, the risk of stroke did not differ between the two study groups.
- The incidence of BARC type 2–5 bleeding was lower in the 6-month DAPT group (2·7% vs. 3·9%) vs. the 12-month or longer DAPT group (HR 0·69 [95% CI 0·45–1·05]; p=0·09).
- As per a post hoc landmark analysis, the risk of major adverse cardiac and cerebrovascular events between 6 months and 18 months was higher in the 6-month DAPT group vs. the 12-month or longer DAPT group (HR 1·69 [95% CI 0·97–2·94]; p=0·07). The rate of all-cause death did not differ between the groups, but patients treat with 6-month DAPT group had more frequent MI as compared to those treated with 12-month or longer DAPT group (5·06 [1·46–17·47]; p=0·01). Also, the risk of cardiac death or MI was significantly higher in the 6-month DAPT group vs. the 12-month or longer DAPT group (HR 2·47 [95% CI 1·14–5·37]; p=0·02). The incidence of BARC type 2–5 bleeding was similar in the 6-month DAPT group and the 12-month or longer DAPT group (HR 0·57 [95% CI 0·29–1·12]; p=0·10).
- The per-protocol analysis and the intention-to-treat analysis yielded similar results.
Conclusion
- The 6-month DAPT strategy was associated with a significantly higher incidence of MI with a very wide non-inferiority margin as compared to the 12-month or longer DAPT strategy. Hence, short-term DAPT cannot be considered non-inferior to long-term DAPT in ACS patients undergoing PCI with current generation DES.
- Prolonged DAPT without excessive risk of bleeding should remain the standard of care in ACS patients.
Lancet 2018; 391: 1274–84.