Introduction
The combination of inhaled corticosteroid and long acting beta-2 agonist (ICS/LABA) is the cornerstone of the treatment of asthma, recommended by GINA and ICS/NCCP guidelines. Fluticasone propionate/formoterol fumarate (FF), a promising ICS/LABA, has been established for its safety and efficacy in the treatment of asthma through several randomized clinical trials. However there is no data available on the efficacy and safety of FF in the real world setting. Revolizer is a single dose dry powder inhaler device with moderate resistance and requires simple inhalation technique.
Aim
To assess the effectiveness of FF delivered twice daily via Revolizer in patients with persistent asthma in a real world setting.
Methods
Study design
- 24-week, open-label, prospective, multicenter, non-comparative, real-world observational study
Inclusion Criteria
- The patients participating in this study were 18 years and above
- Participants were either already on FF via Revolizer or were uncontrolled on other treatments via the Revolizer and required a change in their treatment to FF, as per treating physicians’ discretion
Exclusion criteria
- Patients with hypersensitivity to study drug, drug-drug interactions with FF and pregnant women
Endpoints
Primary endpoints
- Mean change in the level of asthma control using Asthma Control Test (ACT) at week 24.
Secondary endpoints
- ACT scores were also measured at weeks 4, 8 and 16
- Morning and evening peak expiratory flow rate (PEFR)
- Daytime and nighttime symptom scores
- Safety and tolerability
Results
- Out of 401 patients enrolled with a mean age of 41.1+14.36 years, 34.91% patients had mild asthma, 47.88% had moderate asthma and 17.21% had severe asthma. 385 patients completed the study
- The cohort comprised of 202 males and 199 females
- 31.42% had a family history of asthma
- The average smoking pack years was 4.8+3.0 years
- There was a significant improvement in asthma control a week 24.
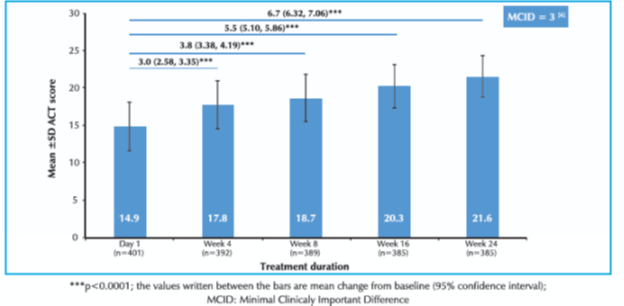
- The mean change in ACT score was 6.7 (p<0.0001) as shown in figure 1.
- Significant increase in mean ACT score was seen from week 4 onwards
- The proportion of patients achieving asthma control increased from 8.5% at baseline to 80.78% at week 24 as seen in figure 2.
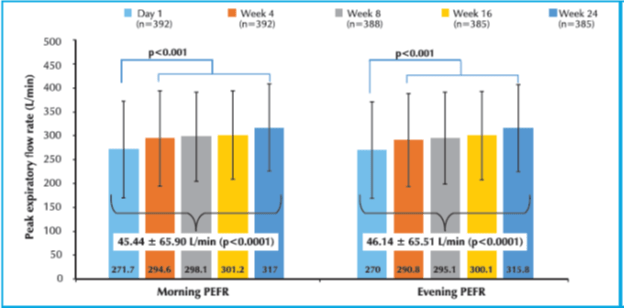
- There was a significant increase of 45.44+65.90 L/min (p<0.0001) and 46.14+65.51 L/min (p<0.0001) in the morning and evening PEFR at week 24 respectively as compared to baseline as shown in Figure 3.
- There was a continuous increase in the number of patients from baseline to week 2, who had no daytime symptoms and no night-time symptoms during the week before the assessments
- Daytime and night-time symptom scores were also reduced significantly (p<0.0001) over 24 weeks.
- Overall, the FF treatment was well tolerated, one patient was hospitalized.
- The following adverse events were reported in >2 patients
- Abdominal pain
- Pain
- Headache
- Urinary tract infection
- Worsening of asthma
- Cough
- Allergic rhinitis
- Oropharyngeal pain
- Pyrexia
Conclusion
In this observational real world study, twice daily Fluticasone/Formoterol administered via Revolizer significantly improved asthma control, peak flow and quality of life of patients with persistent asthma and was well tolerated.
References
- Global initiative of Asthma (GINA), Global strategy for asthma management updated 2015.
- Lung India 2015; Apr 32 (Suppl 1): S3-S42.
- Respir Med 2013; 107:180-195.
- J Asthma 2012; 49(10):1060-70.
- Lung India 2014;31(4):366-374.
- American Academy of Allergy, Asthma and Immunology 2009. 719-723.