To determine the efficacy and safety of 35 mg once-a-week risedronate in men with osteoporosis.
Once-Weekly Risedronate in Men with Osteoporosis: Results of a 2-Year, Placebo-Controlled, Double-Blind Multicenter Study
Once-Weekly Risedronate in Men with Osteoporosis: Results of a 2-Year, Placebo-Controlled, Double-Blind, Multicenter Study
Aim
Materials & Methods
Study design- Multinational, 2-yr, randomized, double-blind, placebo-controlled trial.
N=283
Dosing Regimen
Patients were randomized in a 2:1 ratio to take
Risedronate 35 mg (N = 191) once a week or
Placebo (N = 93) once a week
Patients were given daily supplementation of calcium (1000 mg elemental calcium) and vitamin D (400-500 IU)
Inclusion Criterion
Men ≥30 yr of age
Lumbar spine T-score <-2.5 and femoral neck T-score <-1 SD or lumbar spine
T-score <-1 and femoral neck T-score <-2 SD.
Assessment Parameters
Percent change from baseline in lumbar spine and proximal femur BMD at months 6, 12, and 24
Percent change from baseline in Bone Turnover Markers (BTMs) (CTX, NTX/Cr, BALP) at months 3, 6, 12, and 24
Percent of BMD responders (i.e., those patients who had a positive change in lumbar spine BMD at month 24)
Results
Mean percent change from baseline in BMD :
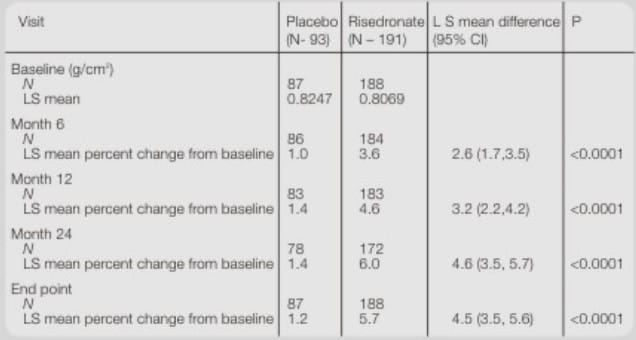
(A) Lumbar Spine BMD (Primary Efficacy End Point)
Lumbar Spine BMD: Least square mean percentage change from baseline at months 6,12 and 24 and end point
Risedronate 35 mg once a week had a statistically significant increase in lumbar spine BMD as compared with placebo, the mean difference being 4.5% at end point.
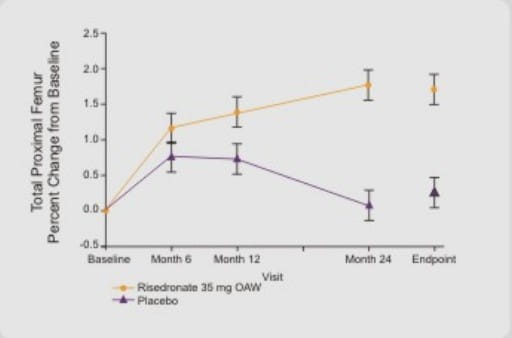
(B) Total Proximal Femur BMD
Risedronate 35 mg once a week resulted in statistically significant increases in total proximal femur BMD at months 12 and 24 and endpoint.
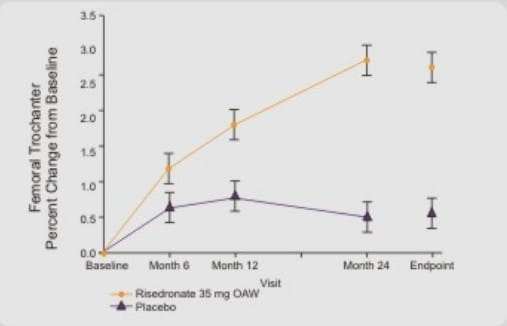
(C) Femoral Trochanter BMD
Risedronate 35 mg once a week resulted in statistically significant increases in femoral trochanter BMD at months 12 and 24 and endpoint.
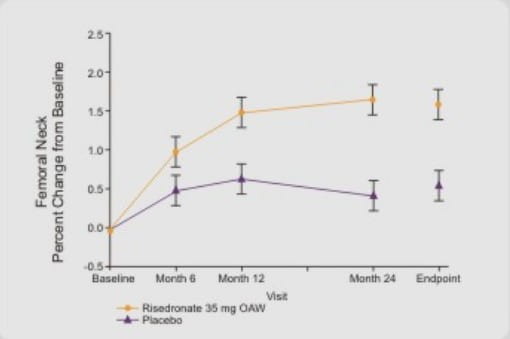
(D) Femoral Neck BMD
Significantly greater increases in femoral neck BMD were observed at month 24 and endpoint in the risedronate 35 mg once a week group compared with placebo.
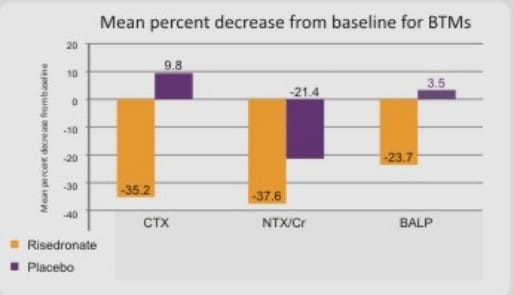
Mean Percent Decrease From Baseline for BTMS (CTX, NTX/CR, BALP)
The mean percent decrease from baseline for each of the BTMs (CTX, NTX/Cr, BALP) was greater (p < 0.0012) for the risedronate group compared with the placebo group at the earliest time point tested (month 3) and at all subsequent time points (months 6, 12, and 24 and endpoint)