Once-Weekly Dulaglutide improves Glycemic Control in Youths with Type 2 Diabetes Mellitus
6 Jul, 22
Introduction
The incidence of type 2 diabetes mellitus (T2DM) is on rise in youths. Once weekly
treatment with dulaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1RA), may be effective in achieving glycemic control in youths with T2DM.
Aim
To ascertain the efficacy and safety of dulaglutide, compared with placebo in youths with T2DM.
Patient Profile
- Patients aged 10 to <18 years with a body-mass index (BMI) >85th percentile for age and sex in the participant’s country, body weight of at least 50 kg, and a glycated hemoglobin (HbA1c) >6.5% to ≤11.0% (if patient managed on metformin with or without insulin) or an HbA1c >6.5% to ≤9.0% (if patient managed on diet and exercise alone) were included in the study.
- Lifestyle measures (diet and exercise) were to be in place for at least 8 weeks before screening. Similarly, metformin and insulin doses had to be stable for at least 8 weeks before screening.
Methods
Study Design
- A phase 3, randomized, placebo-controlled, parallel-group, superiority trial conducted across 46 centers in nine countries
Treatment Strategy
- During the 26-week trial, 154 patients were randomized 1:1:1 to receive once-weekly subcutaneous injections of placebo, dulaglutide at a dose of 0.75 mg, or dulaglutide at a dose of 1.5 mg.
- For patients randomized to 1.5 mg dulaglutide, the medication was initiated at a dose of 0.75 mg for 4 weeks and then escalated to 1.5 mg in absence of any unacceptable adverse events
- Participants were then included in a 26-week open-label extension study wherein patients in placebo group started receiving dulaglutide at a weekly dose of 0.75 mg.
Outcomes
Primary Outcome
- Change from baseline to week 26 in the HbA1c level
Secondary Outcomes
- Percentage of patients achieving HbA1c < 7.0%
- Changes from baseline to week 26 in the fasting plasma glucose (FPG) concentration and BMI
Safety Outcomes
- Safety was also assessed during the study
Results
- Of the entire study population, 95% (n=146) completed the 26-week double-blind period and 90% (n=139) completed the 52-week open-label treatment period.
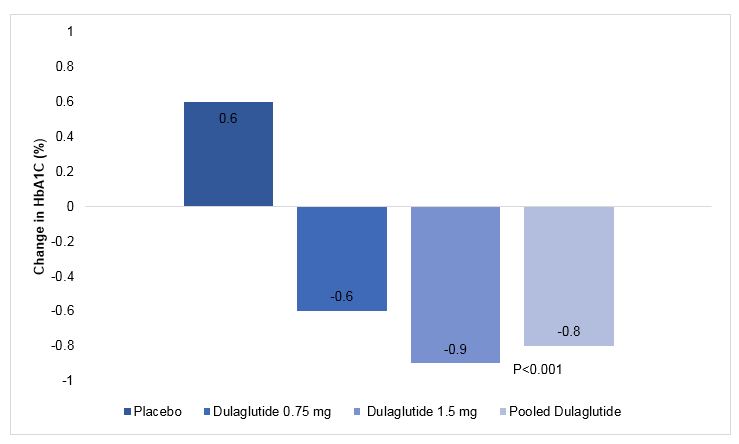
- At 26 weeks, patients in the placebo group had increased mean HbA1c, patients treated with dulaglutide had decreased mean HbA1c (Fig. 1). This reduction continued through the 52 weeks, although the magnitude of reduction was lesser compared to that at 26 weeks.
Fig. 1: Change in the mean HbA1C level during the study period
- At 26 weeks, a greater proportion of patients in the pooled dulaglutide groups vs. the placebo group achieved an HbA1c level <7.0% (51% vs. 14%, P<0.001).
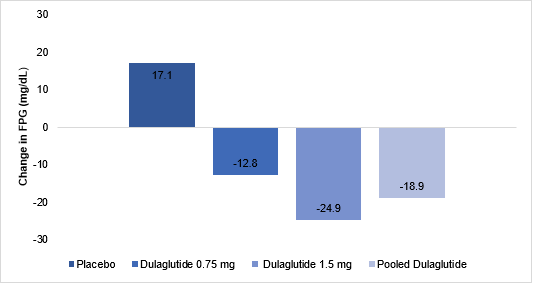
- Patients in the placebo group had increased FPG and those in the pooled dulaglutide group had decreased FPG (Fig. 2)
Fig. 2: Change in FPG during the study
- The incidence of gastrointestinal adverse events was higher in patients treated with dulaglutide vs. placebo. The safety profile of dulaglutide in youths was consistent with that reported in adults.
- There were no clinically relevant between group differences in the change in BMI through 26 weeks
Conclusions
- Once-weekly dulaglutide at a dose of 0.75 mg or 1.5 mg was superior to placebo in improving glycemic control through 26 weeks among youths with inadequately controlled T2DM, without an effect on BMI.
- Improvement in glycemic control due to treatment with dulaglutide persisted through 52 weeks.
New Engl J Med. June 4, 2022 (Published Online); DOI: 10.1056/NEJMoa2204601.










.webp?updated=20240527063817)