Introduction
Earlier in the 1940s, sulfasalazine was prescribed in a conventional dosing schedule for the treatment of ulcerative colitis (UC), a chronic inflammatory disease involving the mucosa of colorectum. Later, a minimum of twice daily (BD) mesalazine was used as it was associated with a reduced toxicity and side effects. However, a divided-dosing regime might result into noncompliance. New formulations of mesalazine with a convenient once daily (OD) dosage regime were developed. The efficacy and safety between OD and multiple daily mesalazine have been evaluated in 3 reviews. However, there was no meta-analysis which assessed the efficacy and safety between OD regime dosing of mesalazine and BD regime.
Aim
This meta-analysis assessed the efficacy and safety between OD and BD regime dosing of mesalazine for mild-to-moderate ulcerative colitis (UC).
Method
Study Design
- Meta-analysis of randomized-controlled trials (RCTs)
Treatment Strategy
- Extensive search of PubMed, Embase, the Cochrane library, and Web of Science databases identified RCTs published from 1990 to November 2018, comparing OD with BD regime dosing of mesalazine for mild-to-moderate UC. The software Review Manager 5.3 was used to pool the risk ratio (RR) with a 95% confidence interval (CI).
Inclusion Criteria
- RCTs that compared OD with BD dosing regimes of mesalazine
- Patients >18 years with confirmed clinical, endoscopic, and histological diagnosis of UC
- Outcomes to include one of clinical and endoscopic remission, clinical remission, mucosal healing, and adverse events (AEs)
Endpoints
Primary Endpoint
- Clinical and endoscopic remission of OD vs BD mesalazine
Secondary Endpoints
- Clinical remission
- Total AEs
- Treatment related AEs
- Serious AEs
Results
- A total of 8 studies including 3495 patients were selected, of whom 1731 (49.5%) patients were randomized in OD mesalazine group and 1764 (50.5%) in BD mesalazine group.
- The intention-to-treat population (ITT) was 3375, of whom 1667 (49.4%) were in OD mesalazine group and 1708 (50.6%) in BD mesalazine group.
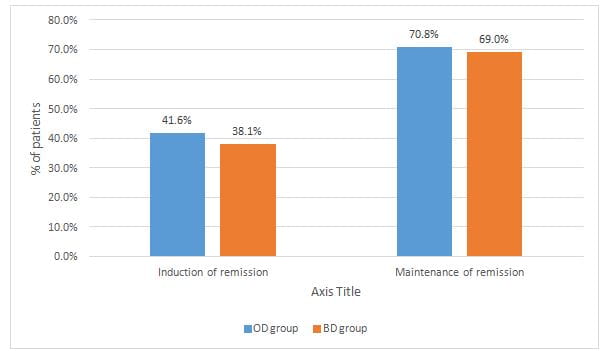
- A pooled analysis with respect to induction of remission implied that there was no notable difference in clinical and endoscopic remission rates between the two treatment groups (RR = 1.08, 95% CI: 0.74–1.57, P = .71) as seen in Figure 1.
- There was no significant difference in clinical and endoscopic remission rate between OD and BD mesalazine group with respect to maintenance of remission (RR = 1.03, 95% CI: 0.94–1.12, P = .58) has seen in Figure 1.
- Similar results were found with regard to the clinical remission (induction of remission, OD vs BD, 39.5% vs 39.2%, P = 0.95; maintenance of remission, OD vs BD, 81.8% vs 79.1%, P = 0.42).
- The total adverse events, treatment-related adverse events or serious adverse events were comparable between the groups, indicating that OD regime dosing was as safe as BD regime dosing of mesalazine.
Conclusion
- The efficacy and safety of once-daily (OD) dosing of mesalazine was comparable to twice-daily (BD) dosing in patients with ulcerative colitis.
- Since ulcerative colitis requires long-term treatment, OD dosing regime can be more convenient than BD dosing regime.
Medicine (Baltimore). 2019 Apr;98(14):e15113.