Montelukast Combined with Mometasone Furoate Offers Superior Clinical Outcomes in Children with Adenoid Hypertrophy
Introduction
Adenoid hypertrophy (AH) has various clinical manifestations depending on the adenoid size. Apart from chronic or recurrent infections, AH can result in severe complications when the nasal airway gets obstructed. Intranasal corticosteroids such as mometasone furoate (MF) reduces adenoid size and improves the quality of life in children with AH. Montelukast, an oral cysteinyl leukotriene receptor antagonist, has also been studied for the treatment of AH.
Aim
The efficacy and recurrence rate of combined therapy using montelukast and intranasal MF has been compared to intranasal MF alone in children with AH.
Methods
Study Design
- Prospective randomized study.
Patient Profile
- Children aged 3-10 years diagnosed with AH by lateral neck radiographic examination
- Adenoid/Nasopharynx ratio (A/N ratio >50%)
- AH grade 3 or 4
Treatment Strategy
- After undergoing a thorough clinical assessment, the eligible children were randomized into 2 groups
- Group I (50 patients) received combined therapy using montelukast (4 mg once daily in children <6 years and 5 mg in children >6 years) and MF nasal spray (2 puff, total 100 µg in each nostril, once daily).
- Group II (50 patients) received only MF intranasal spray.
- The children underwent treatment for 3 months and observed for 3 months after cessation of treatment to detect the recurrence rate.
- The cohort was assessed using symptoms scores, A/N ratio and endoscopic grading of AH.
Endpoints
- Change in symptom score
- Change in AH grade
- A/N ratio
- Recurrence rate
Results
- The baseline characteristics of both the groups were similar.
- The group 1 demonstrated significantly better scores of the main symptoms after 3 months of treatment than group II; (P = 0.001), (P = 0.019) and (P = 0.008) for rhinorrhea, mouth breathing and snoring respectively.
- After further 3 months of cessation of treatment, significant better scores of the main symptoms were observed for group 1 (p<0.001 for all symptoms).
- The mean A/N ratio was also significantly better in group 1 post-treatment and after further 3 months of stoppage of treatment (p<0.001) as shown in Table 1.
|
|
Baseline |
3 months post-treatment |
3 months after cessation of treatment |
|
Group 1 |
67.48+6.02 |
52.8+11.3 |
58.46+10.05 |
|
Group 2 |
69.34+7.85 |
62.88+12.10 |
66.36+10.46 |
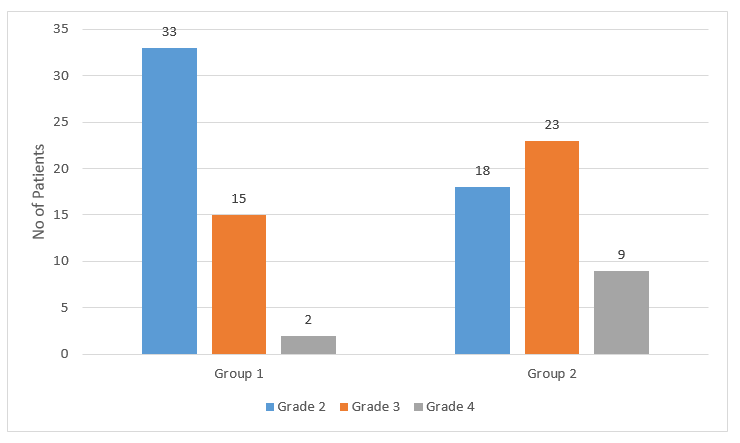
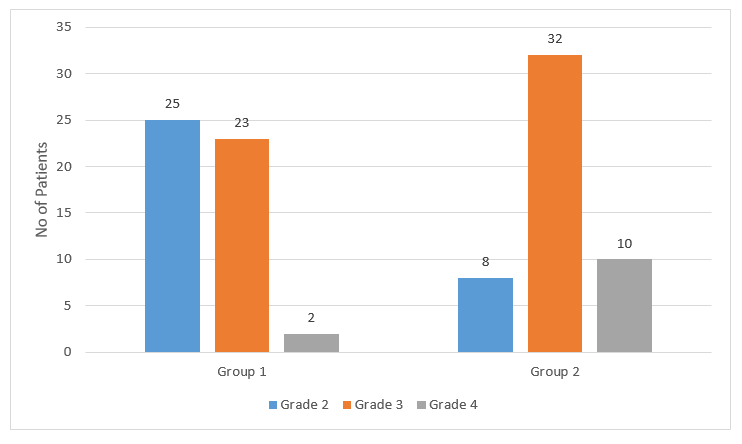
- Group 1 showed better improvement with respect to endoscopic grading of AH at the end of 3 months of treatment as seen in Figure 1.
- There was a significant difference in the endoscopic grading in both groups, with better results in group 1, at 3 months after cessation of treatment.
- Group 1 had a significantly lower recurrence rate as compared to group 2 (23.5% vs 55.5%; p = 0.02).
Conclusion
- Combined therapy with oral montelukast and intranasal mometasone resulted in significant improvements with lesser recurrence in children with adenoid hypertrophy as compared to single therapy with intranasal mometasone alone.
Am J Otolaryngol. Nov-Dec 2020;41(6):102723.