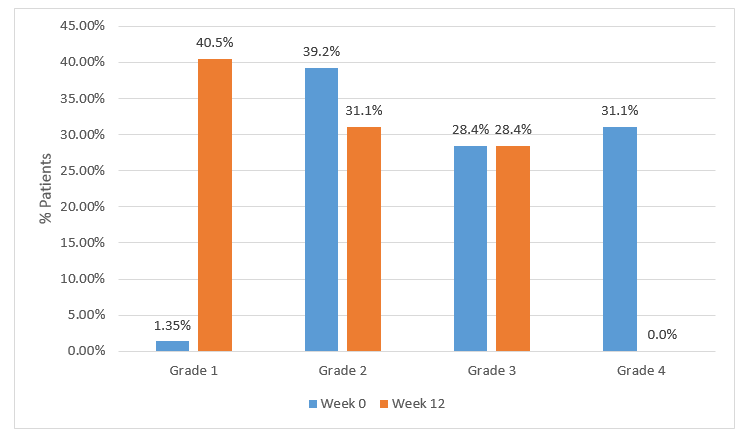Mometasone Furoate Improves Symptoms and Reduces Adenoid Size in Children and Adolescents with Adenoid Hypertrophy
2 Feb, 22
Introduction
Several studies have demonstrated that mometasone furoate (MF) intranasal spray reduces symptoms, reduces adenoid size and improves the quality of life in children and adolescents with adenoid hypertrophy (AH). However, different methods have been used by these studies to assess the adenoid size leading to variable outcome.
Aim
This study uses a reliable and consistent endoscopic grading assessment to evaluate the effect of MF intranasal spray in children and adolescents with AH.
Methods
Study Design
- Prospective interventional study.
Patient Profile
- Patients with symptoms of AH aged 7-17 years
- Adenoid size grade 2-4
Treatment Strategy
- The patients underwent clinical examination to assess symptoms such as nasal obstruction, rhinorrhea, coughing and snoring.
- Nasal endoscopy was conducted at enrollment based on four-grading system of adenoid size from 1 to 4.
- Evaluation was done at week 0 and week 12.
- The cohort was treated with MF intranasal spray for 12 weeks.
- Children aged 7-11-years old used 1 spray in each nostril once daily, while patients aged 12-17 used two sprays in each nostril once daily.
- The efficacy of MF nasal spray was assessed at second visit (week 12).
Endpoints
- Individual symptom score
- Total nasal symptom score (TNSS)
- Adenoid size
Results
- The study population included a total of 74 patients.
- Significant reductions in individual symptom scores as well as TNSS was observed from week 0 to week 12 (p<0.001 for all) as seen in Table 1.
Table 1. Assessment of outcomes at week 12.
|
Symptoms |
Mean scores at week 0 |
Mean scores at week 12 |
Mean score difference |
|
Nasal obstruction |
2.04 |
1.07 |
0.97 |
|
Rhinorrhea |
2.26 |
1.18 |
1.08 |
|
Cough |
1.01 |
0.26 |
0.76 |
|
Snoring |
1.69 |
0.80 |
0.89 |
|
TNSS |
7.00 |
3.31 |
3.69 |
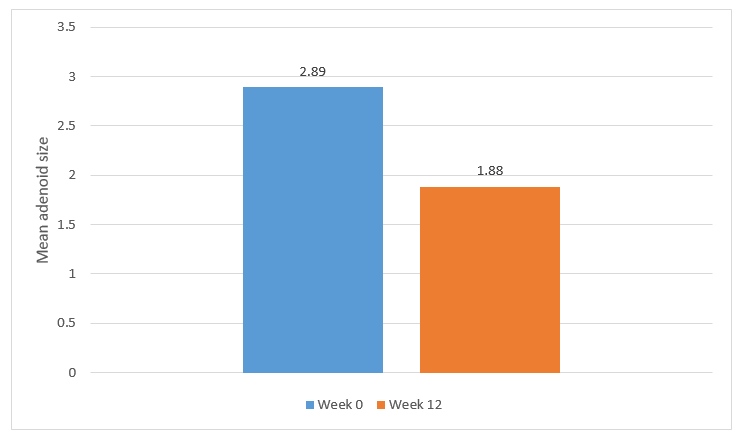
- The adenoid size reduced remarkably post-treatment; mean treatment difference 1.01, p<0.001 as seen in Figure 1.
Figure 1. Adenoid size pre- and post-treatment
- None of the patients had grade 4 at week 12.
- The distribution of patients based on the endoscopic grading system pre- and post-treatment is compared in Figure 2.
Figure 2. Distribution of patients based on endoscopic grading pre- and post-treatment
Conclusion
- Mometasone furoate (MF) nasal spray was found to significantly improve the symptoms attributed to adenoid hypertrophy (AH) as well as reduce the adenoid size in children and adolescents.
- MF intranasal spray can be a good treatment option before adenoidectomy is considered.
Acta Otorrinolaringol Esp (Engl Ed). May-Jun 2020;71(3):147-153.