Intravenous Colistin Effective and Safe in Very Low Birth Weight Preterm Infants
1 Feb, 21
Introduction
The recent years have witnessed a surge in multidrug-resistant Gram-negative bacilli (MDR-GNB) infections, nevertheless; there are limited antimicrobials that can treat these infections. Consequently, the role of colistin for treating MDR-GNB infection has been increasingly reconsidered. Currently there is very limited clinical data on the efficacy and safety of intravenous (IV) colistin in neonates.
Aim
To compare the efficacy and safety of IV colistin in very low birth weight (VLBW) preterm infants vs. more mature infants with MDR-GNB infections
Patient Profile
- Infants who were administered IV colistin for > 72 h due to MDR-GNB infections (age; 4-120 days)
- The patients were categorized in the following two groups:
- VLBW group (Birth Weight < 1500 g)
- Non-VLBW group (infants with birth weight > 1500 g)
Methods
Study Design
- Retrospective single centre study
Treatment Strategy
- Infants identified as having MDR-GNB infection (based on culture reports and an outbreak of blood stream infection caused by MDR-Klebsiella pneumonia) received IV colistin 5 mg/kg/day in 3 divided doses as an infusion in 5 ml saline over at least 30 min
- The colistin formulation comprised of 150 mg of colistin base-equivalent to Colistimethate Sodium, with 1 mg of colistimethate sodium being equivalent to 30,000 IU
Outcomes
- Response to treatment (based on clinical and microbiological response)
- Adverse effects
Results
- A total of 1260 infants were admitted to neonatal intensive care unit (NICU), of these; 152 (12%) were diagnosed with neonatal sepsis and 72 of them were administered IV colistin.
- This analysis included 66 infants who received colistin therapy, 28 infants (42.4%) were VLBW and 38 (57.6%) were non-VLBW. Median duration of colistin treatment was 14 days.
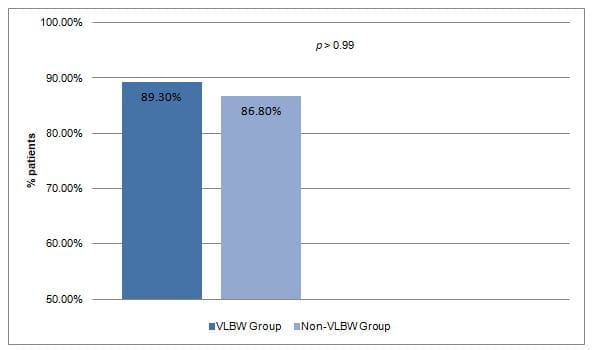
- The efficacy of colistin (defined as showing microbiological clearance in control cultures and the absence of mortality during treatment) did not differ significantly in the study groups (Fig 1).
Fig 1: Efficacy of colistin the study groups
- Mortality due to Klebsiella spp infection did not differ significantly in both the study groups (P=0.35).
- Incidence of acute kidney injury (AKI) was higher the VLBW group vs. the non-VLBW group, but the difference was not significant statistically (4 cases vs. 1 case, p=0.15).
- During colistin therapy, infants in the VLBW group had significantly lower levels of serum magnesium and potassium vs. the infants in the non-VLBW group (magnesium, 1.30 vs 1.70 mg/dL, p < 0.001; potassium, 3.6 vs 4.6 mEq/L, p < 0.001)
Conclusions
- Treatment with IV colistin appeared to be effective in VLBW infants with MDR-GNB.
- Nevertheless, colistin may hamper postnatal kidney development in preterm infants, hence; close monitoring of renal function and serum electrolytes is essential in these infants during treatment.
Pediatr Drugs. 2018; 20:475–481.
Related Topics