Intranasal Spray Containing Azelastine hydrochloride and Fluticasone propionate is Safe and Well Tolerated in Children with Allergic Rhinitis
Introduction
Nasal allergy symptoms have an adverse impact of the physical and emotional health in children. There is a need for a new option in the treatment of allergic rhinitis (AR) that can provide rapid symptom relief and is safe in children below 12 years old. The novel intranasal formulation contains azelastine hydrochloride and fluticasone propionate delivered in a single spray. The efficacy and safety of this formulation has been established in adults and adolescents. Few studies have demonstrated significant symptom relief and improvement in the quality of life (QOL) with this unique formulation in children aged 4 to 11 years. However, its safety and tolerability in pediatrics is yet to be established.
Aim
The safety and tolerability of a combined intranasal formulation containing azelastine hydrochloride (AZE) and fluticasone propionate (FP) was evaluated in children aged 4-11 years with AR.
Methods
Study design
- Prospective, randomized, multi-center, active-controlled, open-label, parallel-group study
- Children aged 4-11 years with AR; seasonal AR (SAR) or perennial AR (PAR)
- Randomized 3:1 to receive either combined formulation containing 137 µg AZE and 50 µg FP (n=304) or FP nasal spray (n=101); one spray per nostril twice daily
- Children were classified into 3 groups - >4 to <6 years, >6 to <9 years and >9 to <12 years
Endpoints
- Child- or caregiver-reported adverse events (AEs)
- Nasal examinations
- Vital signs
- Laboratory assessments
Results
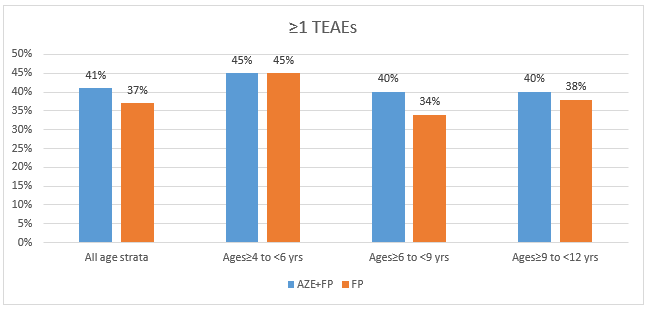
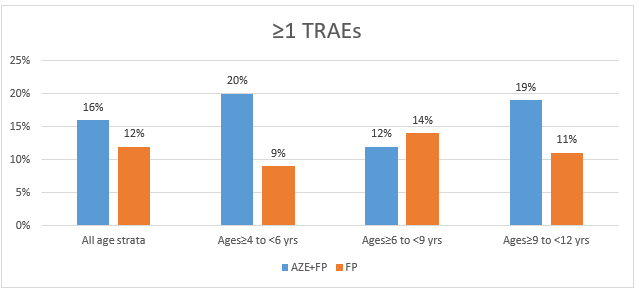
- The incidence of treatment-emergent AEs (TEAEs) and treatment-related AEs (TRAEs) across all the age groups were similar in both arms as seen in figures 1 and 2 respectively
- All the TEAEs and TRAEs were mild in severity
- There were no deaths reported
- Epistaxis was the most common TRAE reported by 9% in each arm
- Headache was reported by 3% in AZE+FP arm and by 1% in the FP arm
- The severity of nasal symptoms gradually reduced over time in both the arms
- There was no incidence of mucosal ulceration or nasal septal perforation
- There were no unusual changes in the vital signs and laboratory assessments
Conclusion
- The combined intranasal formulation containing azelastine hydrochloride and fluticasone propionate was safe and well tolerated after 3 months continuous use in children aged 4 to 11 years with allergic rhinitis (AR)
- This novel formulation might be a good option in the treatment of moderate to severe AR in pediatrics
Allergy Asthma Proc. 2018 Mar 1;39(2):110-116. Doi: 10.2500/aap.2018.39.4116.