HOPE 3: Long-term Cholesterol Lowering with Low-dose Rosuvastatin Alleviates CV Risk in Intermediate-Risk Multi-Ethnic Population
5 Jan, 17
Introduction
The role of statins in reducing the risk of cardiovascular (CV) events among persons with cardiovascular disease (CVD) is well-established. It is however not known whether the statin treatment would also be beneficial for individuals without CVD, regardless of lipid levels, inflammatory markers, hypertension status, or diabetes status.
Aim
The Heart Outcomes Prevention Evaluation (HOPE)–3 trial was conducted to evaluate the long-term effects of rosuvastatin in intermediate-risk, multi-ethnic population without CVD.
Patient Profile
- Men (age ≥ 55years) and women (age ≥ 65 years) with at least one CV risk factor (elevated waist-to-hip ratio, history of a low level of high-density lipoprotein cholesterol, current/ recent tobacco use, dysglycaemia, family history of premature coronary disease, and mild renal dysfunction)
- Women aged ≥ 60 years with at least 2 of the above CV risk factors
- Around 49.1% of the study population was Asian, 20% subjects were white, 27.5% were Hispanic, and 3.3% were black or belonged to another ethnic group
Methods
Study Design
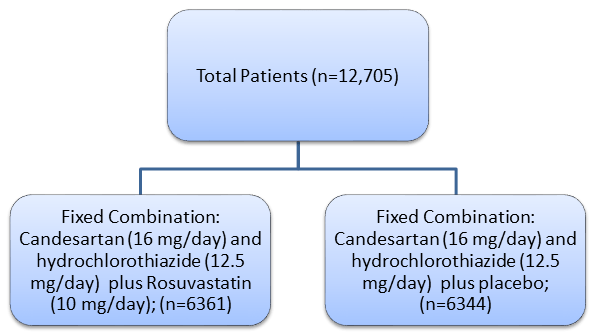
- Pragmatic, multicentre (228 centers across 21 countries) long-term, international, double-blind, randomized, placebo-controlled trial with a 2-by-2 factorial design that evaluated cholesterol lowering with rosuvastatin (10 mg/ day,) blood-pressure lowering with candesartan (16 mg/ day) plus hydrochlorothiazide (12.5 mg/day), and the combination of both interventions for the prevention of CV events in the study subjects.
Treatment Strategy
Single Blinded Run-in Phase
- Active BP lowering and cholesterol lowering treatment for 4 weeks
- Treatment adherent patients with no unacceptable levels of adverse events (AEs) randomized to one of the following:
Follow-up
- At 6 weeks and 6 months after randomization and every 6 months thereafter
- Median follow-up period: 5.6 years
Primary Outcome
First Coprimary Outcome:
- Composite of death from CV causes, nonfatal myocardial infarction, or nonfatal stroke
Second Coprimary Outcome:
- Resuscitated cardiac arrest, heart failure, and revascularization in addition to the first coprimary outcome
Secondary Outcome
- Second coprimary outcome plus angina with evident ischemia
Results
- Patients in the rosuvastatin group had 26.5% (34.6mg/dL) lower mean low-density lipoprotein cholesterol level vs. the placebo group
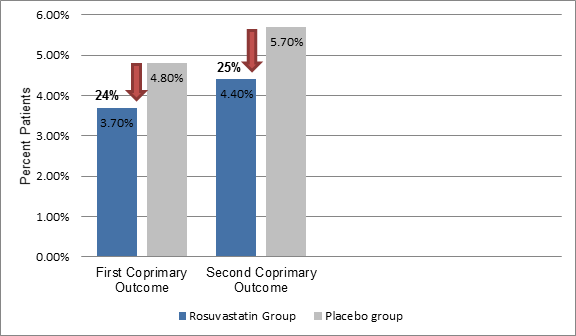
- The incidence of first coprimary outcome was 24% lower (3.7% vs. 4.8%, hazard ratio [HR]; 0.76, P = 0.002) and that of second coprimary outcome (4.4% vs. 5.7%, HR; 0.75; P<0.001) was 25% lower in the rosuvastatin group vs. the placebo (Figure 1)
Figure 1: The incidence of primary outcomes in the study group
- The results remained consistent in subgroups defined as per the CV risk at baseline, lipid level, C-reactive protein level, BP, gender, age, and race or ethnic group.
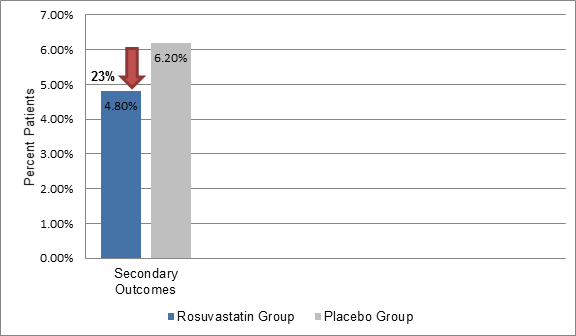
- The incidence of secondary outcome was also 23% lower in the rosuvastatin group vs. the placebo group (4.8% vs. 6.2%, HR; 0.77, P<0.001) (Figure 2)
Figure 2: The incidence of secondary outcomes in the study group
- Significantly fewer patients on rosuvastatin experienced stroke, particularly, ischemic stroke (41 patients vs. 77) as compared to those on placebo
- The incidence of hemorrhagic stroke was slightly higher in rosuvastatin users
Adverse Events
- Greater proportion of patients using rosuvastatin experienced muscle pain or weakness (5.8% vs. 4.7%; p=0.005) vs. placebo
- There was no significant difference in the discontinuation rates or the incidence of rhabdomyolysis or myopathy in both the groups
- Although there was no excessive incidence of diabetes or cancers, more participants in the rosuvastatin group underwent cataract surgery as compared to the placebo group (3.8% vs. 3.1%; P = 0.02)
Conclusion
- Cholesterol lowering with low-dose rosuvastatin (10 mg/day) for a period of 5.6 years significantly lowered the risk of CV events in an intermediate-risk, multi-ethnic population without CVD and having lipid levels in normal range
N Engl J 2016; 374 (21): 2021-31.