Efficacy and Safety of Once-Daily Combination of Fluticasone Furoate and Vilanterol in Asthma Control
Introduction
The guidelines for management of asthma are mainly based on the efficacy randomized controlled trials (RCTs) in highly selected and closely monitored patient populations, usually excluding patients with a smoking history and comorbidities. These may have limited relevance in everyday clinical practice and hence there is a need of comparative clinical studies done in more representative patients in usual care settings. The Salford Lung Studies were designed to assess the efficacy and safety of once-daily inhaled combination of fluticasone furoate and vilanterol compared with continuation of maintenance therapy (usual care) in a real-world population with COPD (chronic obstructive pulmonary disease) and asthma.
Aim
The Salford Lung Study on asthma was conducted to compare the efficacy and safety of inhaled combination of fluticasone furoate and vilanterol with the optimized usual care.
Method
Study Design
- Prospective, 12-month, open-label, parallel-group, randomized study done at 74 general practice clinics in Salford and South Manchester, UK.
Patient Profile
- Patients <18 years diagnosed with asthma by a general practitioner
- Patients on regular maintenance therapy with inhaled corticosteroid (ICS) alone or in combination with a long-acting ?-agonist (LABA).
Treatment Strategy
- The patients were randomized to receive a once-daily inhaled combination of either 100 ?g or 200 ?g fluticasone furoate with 25 ?g vilanterol as a dry powder through inhaler or optimized usual care with maintenance therapy comprising ICS alone or in combination with LABA.
- The patients were assessed for their asthma control, smoking history, comorbidities, etc. and were followed up for 12 months.
- All effectiveness analyses were done according to the intention-to-treat principle.
End Points
Primary Endpoints
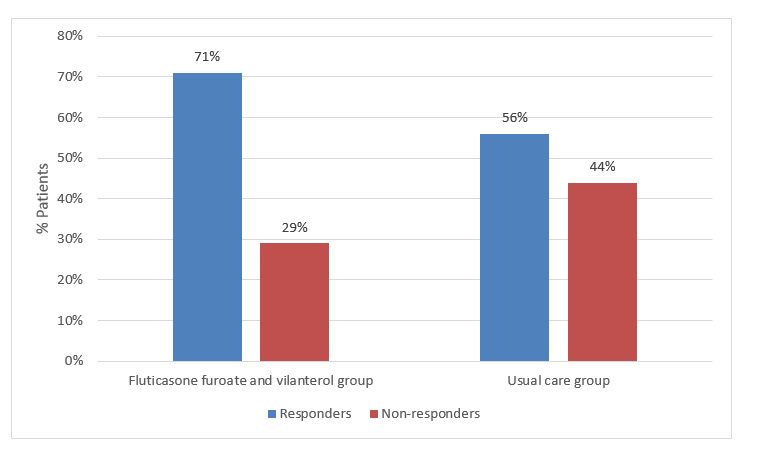
- % Patients who achieved an asthma control test (ACT) score >20 or an increase in ACT score from baseline >3 at 24 weeks (termed responders), in patients with a baseline ACT score <20 (the primary effectiveness analysis population).
Secondary Endpoints
- Mean annual rate of severe exacerbations
- % Patients with increase in baseline of atleast 0.5 in total asthma quality-of-life questionnaire (AQLQ)
Safety Endpoints
- Serious adverse events of pneumonia
- Other serious adverse events (SAEs)
Results
- Out of the total of 4725 patients, 4233 patients were randomly assigned to initiate treatment with fluticasone furoate and vilanterol (n=2114) or usual care (n=2119).
- A total of 1207 patients (605 assigned to usual care, 602 to fluticasone furoate and vilanterol) had a baseline ACT score >20 and were thus excluded from the primary effectiveness analysis population.
- The primary effectiveness analysis population comprised 1512 in the fluticasone furoate plus vilanterol group and 1514 in the usual care group.
- At week 24, the odds of being a responder to initiation of treatment with fluticasone furoate and vilanterol were twice the odds of being a responder with usual care.
- The % of responders were higher in the fluticasone furoate and vilanterol group, odds ratio (OR) 2·00, p<0·0001; as shown in Figure 1.
- This benefit was consistent across all the subgroups, with no effect of baseline characteristics.
- At week 24, the adjusted mean ACT score increased by 4·4 points from baseline of 14.4 in patients initiated with fluticasone furoate and vilanterol, compared with 2·8 points from 14.2 in the usual care group (difference 1·6; p<0·0001).
- Similar results were observed at weeks 12, 40 and 52.
- The adjusted annual exacerbation rate and time to first exacerbation did not differ significantly among the groups.
- The % of responders based on AQLQ score was significantly higher in the fluticasone furoate and vilanterol group at week 52, OR 1.79; p<0.0001.
- There were no significant differences in the incidence of pneumonia and other SAEs among the groups.
Conclusions
- Once-daily combination treatment of fluticasone furoate and vilanterol resulted in better asthma control without increasing the risk of serious adverse events as compared with maintenance therapy using ICS alone or in combination with LABA.
Lancet. 2017 Nov 18;390(10109):2247-2255. Doi: 10.1016/S0140-6736(17)32397-8