Efficacy and Safety of Colistin Loading Dose in Critically Ill Children, Affected with Nosocomial Infections
23 Dec, 20
Introduction
Pharmacokinetic and clinical studies have recommended the use of loading dose of colistin for treating severe infections in the critically ill adults. Similarly, pharmacokinetic studies of colistin conducted in children have also underscored the need for a loading dose. Nevertheless, no clinical study till date has evaluated the effectiveness of colistin loading dose in children.
Aim
To determine the efficacy and safety of colistin loading dose in critically ill children affected with nosocomial infections due to extensively drug resistant (XDR) Gram-negative bacteria
Patient Profile
Inclusion Criteria
- Critically ill children with ventilator?associated pneumonia or central line?associated bloodstream infection (CLABSI) having a positive specimen with polymyxin-only-susceptible (POS) Gram-negative bacteria (n=44)
Exclusion Criteria
- Patients with previous renal failure (acute or chronic)
- Patients with previous treatment with colistin in the current admission or treatment with other antibiotics targeting Gram-negative bacteria except for meropenem
- Patients receiving colistin for <72 h and/or receiving colistin by inhalation
Methods
Study Design
- A parallel randomized clinical trial
Treatment Strategy
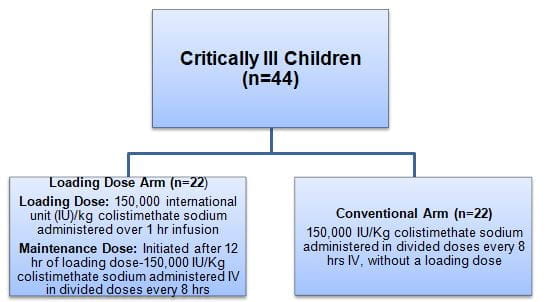
- Patients were randomized as follows:
- Patients in both the study arms also received 30 mg/kg/q8 h IV meropenem as combination therapy with colistin
- For efficacy outcomes, patients were followed from 72 hr after initiation of colistin regimen
Outcomes
Primary outcomes
- Overall efficacy (achieving clinical and microbiological response)
- Clinical improvement (defined as alleviation of signs such as fever, leucocytosis; for VAP-as improvement of all baseline signs and symptoms associated with pneumonia)
- Microbiological response (defined as microbiological clearance of the isolated causative organism)
Secondary outcomes
- Incidence and severity of colistin?induced acute kidney injury (AKI)
- Development of resistance to colistin
Results
- Thirty children completed this study, 14 in the loading dose arm and 16 in the maintenance dose arm.
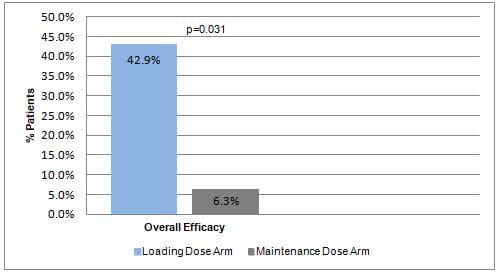
- Patients who received loading dose had a significantly higher overall efficacy as compared to those who did not receive a loading dose (Fig. 1).
- The clinical and microbiological outcomes did not differ significantly between both the study groups.
- Amongst the subgroup of children with CLABSI, a trend towards better clinical cure was evident for patients receiving loading dose, though the change was not significant statistically.
- Acute kidney injury was detected in two patients, one in each group. Both patients had received concomitant nephrotoxic medications with colistin. Supportive treatment successfully reversed AKI.
- No cases of colistin resistance were observed.
Conclusions
- Amongst critically ill children affected with nosocomial infections, 150,000 IU/Kg loading dose of colistin improved overall efficacy without adversely affecting the renal function.
- Colistin loading dose appeared to be beneficial particularly in children with CLABSI.
- In-depth studies that would explore the best dosing regimen for colistin in critically ill children affected with severe infections are warranted.
J Res Pharm Pract 2019;8:196-201.
Related Topics