Combined Effect of Levetiracetam and Lacosamide in Patients with Epilepsy
24 Mar, 21
Introduction
Conventional first-line anti-epileptic drugs (AEDs) effectively control seizures in most of the epilepsy patients, though nearly 30% patients will have persistent seizures. Traditionally, AEDs may have adverse impact on the skeletal system of middle-aged and elderly patients. Levetiracetam and lacosamide (LCM) are two very effective AEDs, but the combination of both the medications has been rarely used for the treatment of senile epilepsy. Moreover, the impact of the combination on the efficacy and neural function in patients is unclear.
Aim
To investigate the effects of levetiracetam tablets and LCM on therapeutic efficacy and neural function in patients with epilepsy
Patient Profile
- Adult patients with refractory partial seizures (n=252)
- Patients experienced more than 4 seizures per month and had a poor response to stable administration of one or more first-line AEDs.
Methods
Study Design
- A prospective study
Treatment Strategy
- Control Group: The study subjects received only levetiracetam tablets (n=120)
- Initial dose and dose escalation: 250 mg, twice a day, further increased to 500 mg, twice a day after two weeks, and then to 1,000 mg, twice a day after another two weeks
- Maintenance dose: The dose of levetiracetam was maintained at 1,000 mg and adjusted as per the patient’s condition
- Joint Group: The study subjects received a combination of levetiracetam and LCM tablets (n=132)
- Initial dose: Levetiracetam (dosage regimen similar to that of the control group) + LCM 50 mg twice a day, increased every week by 100 mg/day based on the patient response
- Maintenance dose: Levetiracetam (the dosage regimen similar to that of the control group) + LCM maintained at 200-400 mg/day.
Outcomes
- The following outcomes were assessed at 6 months before and after treatment:
- Treatment efficacy
- Seizure frequency
- The bone mineral density (BMD) in different body parts
- Serum levels of markers for neural function including Glial fibrillary acidic protein (GFAP), neuron?specific enolase (NSE), and S?100? protein (S?100?)
- Adverse reactions during the treatment
- Quality of life as assessed by the Quality of Life in Epilepsy Inventory (QOLIE?31) score
Results
- The baseline demographics were comparable in the two study groups.
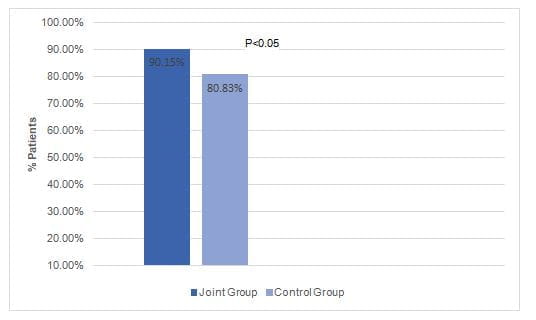
- The total response rate after 6 months was significantly higher in the joint group vs. the control group (Fig. 1).
Fig 1: The total response rate in the study groups
- The seizure frequency decreased in both the groups after 6 months of treatment, though the seizure frequency remained slightly higher in the control group (P<0.05).
- Following the treatment, the levels of neurological indicators reduced significantly in the both the groups (P<0.05), but the reduction was more distinct in the joint group vs. the control group.
- Following the treatment, the BMD of the femoral neck decreased in both the groups (P<0.05), but with no between-group difference (P>0.05).
- Following the treatment, the calcium content decreased in both the groups (P<0.05), with no between-group difference (P>0.05).
- The changes in other bone metabolism markers did not differ significantly between the two groups.
- The incidence of adverse events did not differ significantly between the joint group and the control group (20.46% vs. 25.83%; p> 0.05)
- The one-year drug retention was higher in the joint group vs. the control group (74.24% vs. 66.67%)
- The QOLIE-31 score improved significantly in both the study groups with a slightly higher improvement in the joint group vs. the control group (p<0.05)
Conclusions
- Levetiracetam tablets combined with LCM significantly enhanced the therapeutic effect and improved the neural function in patients with refractory partial seizures.
- The therapy may cause have a slight adverse impact on BMD and bone metabolism in the short term.
- The combination therapy greatly increased the QOL score and the 1-year drug retention rate
Exp Ther Med. 2020 Oct;20(4):3687-3694.