Introduction
Many individual risk factors, such as hypertension and dyslipidemia together synergistically exacerbate the development and severity of cardiovascular disease (CVD). Targeting multiple disease factors with combination treatment may aid in achieving the treatment goals more effectively. A combination of angiotensin receptor blocker (ARB; telmisartan 80 mg) and statin (rosuvastatin 20 mg) and calcium channel blocker (CCB; amlodipine; 10 mg) and rosuvastatin 20 mg have demonstrated a good efficacy and safety profile in patients with concomitant hypertension and dyslipidemia. A combination of ARB, CCB and statin may therefore have an excellent efficacy and safety in patients with hypertension and dyslipidemia.
Aim
To compare the efficacy and safety of telmisartan/amlodipine plus rosuvastatin vs. telmisartan plus rosuvastatin or telmisartan/amlodipine in patients with hypertension and dyslipidemia
Patient Profile
- Drug-free adults (≥19 years) with hypertension [mean sitting systolic blood pressure (MSSBP ≥160 mmHg and <180 mmHg)] and dyslipidemia [low-density lipoprotein cholesterol (LDL-C) level ≤250 mg/dL, and a triglyceride (TG) level?400 mg/dL)] at screening
- The study also included patients with hypertension (MSSBP ≥130 mmHg and ?180 mmHg) and dyslipidemia (LDL-C level of ≤250 mg/dL, TG level ?400 mg/dL) and concomitant diabetes or chronic kidney disease
Method
Study Design
- Randomized, double-blind, active-controlled, multicentre, phase 3 trial
Treatment Strategy
After a 4-week run-in period 202 patients were randomized to 8-week treatment period into following three groups:
- Telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg (T/A+R) group
- Telmisartan 80 mg + rosuvastatin 20 mg (T+R) group
- Telmisartan/amlodipine 80/5 mg (T/A) group
Outcomes
Primary Outcome
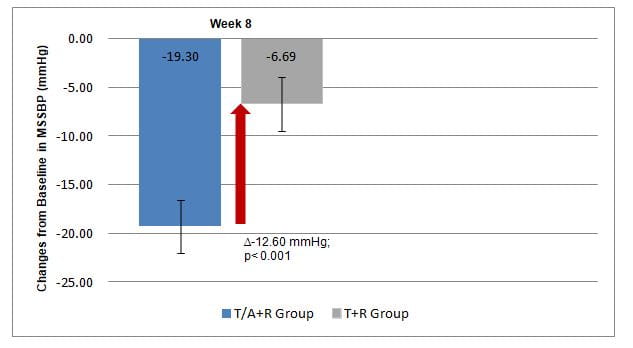
- Changes from baseline in MSSBP between T/A+R and T+R at 8 weeks
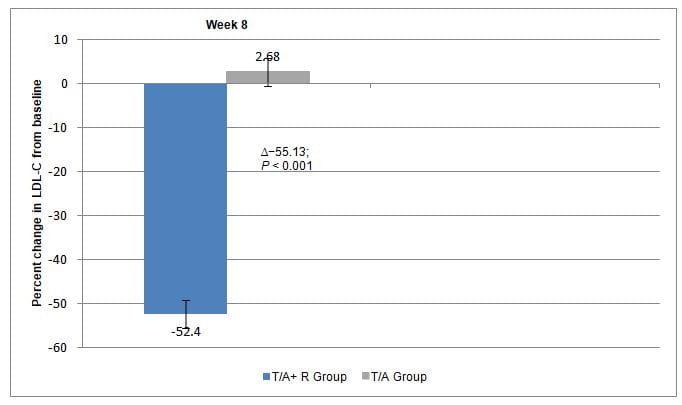
- Percent changes from baseline in LDL-C between T/A+R and T/A at 8 weeks
Secondary Outcomes
- Changes from baseline in MSSBP, mean sitting diastolic blood pressure (MSDBP) at weeks 4 and 8 after treatment initiation
- Percent changes from baseline in LDL-C and other lipid levels at weeks 4 and 8 after treatment initiation compared with baseline
- Adverse events during follow-up
Results
- The demographic and baseline characteristics did not differ significantly between the three study groups.
- The mean overall treatment compliance was strong for all the three groups (98.42%, 96.68%, and 98.12% for T/A+R, T+R and T/A groups, respectively).
- The Least-Square (LS) mean (SE) for changes in MSSBP were significantly greater in the T/A+R group vs. the T+R group (Fig 1).
- The LS mean for the percent changes from baseline in LDL-C were higher with T/A+R vs. T/A (Fig 2).
- The LS mean of the MSSBP changes at week 4 were significantly better in T/A+R group and T/A group than that observed in T+R group. The difference was not significant for T/A+R and T/A groups (Table1).
- With regards to LS mean of MSDBP changes at weeks 4 and 8, the difference was significant between T/A 80/5 mg + R 20 mg and T 80 mg + R 20 mg groups was significant at 4 weeks (−6.27 (1.16) mm Hg, 95% CI −8.56 to −3.98, P < 0.001) and 8 weeks (−6.06 (1.38) mm Hg, 95% CI −8.78 to −3.33, P < 0.001). The difference was not significant for T/A 80/5 mg + R 20 mg and T/A 80/5 mg at all time points.
|
|
T/A+R Group |
T/A Group |
T+R Group |
Difference between T/A+R and T+R groups |
Difference between T/A and T+R groups |
|
LS mean of the MSSBP change at week 4 (mmHg) |
−19.61 |
-13.57 |
−5.66 |
−13.95 (p<0.001) |
-6.04 (p<0.0201) |
|
LS mean of the MSDBP changes at week 4 (mmHg) |
−7.26 |
-7.13 |
−0.99 |
-6.27 (p<0.001) |
Not significant at all time points |
|
LS mean of the MSDBP changes at week 8 (mmHg) |
−7.89 |
-6.31 |
−1.83 |
-6.06 p<0.001) |
Not significant at all time points |
- The LS mean for the percent change from baseline to weeks 4 and 8 in LDL-C was significantly greater in the T/A+R group vs. the T/A group (Table 2).
- Total cholesterol levels decreased at all time points post-administration in the T/A+R group; on the contrary, total cholesterol increased at all time points in the T/A group. Difference for percent change in total cholesterol between the two treatment groups was significant at weeks 4 and 8 (Table 2).
- A significantly higher TG reduction was observed in the T/A+R group vs. the T/A group at weeks 4 and 8 (Table 2).
- HDL cholesterol increased at all time points in both T/A+R and T/A groups, but the increase was higher in the former vs. later group (Table 2).
|
|
T/A+R Group |
T/A Group |
Difference between T/A+R and T+A groups |
|
LS mean for percent change in LDL-C at week 4 |
−51.68% |
2.42% |
−54.10% (p<0.001) |
|
LS mean for percent change in LDL-C at week 8 |
−52.45 |
2.68% |
-55.13% (p<0.001) |
|
LS mean for percent change in total cholesterol at week 4 |
−35.06% |
2.74% |
-37.80% (p<0.001) |
|
LS mean for percent change in total cholesterol at week 8 |
-35.31 |
2.86 |
38.17% (p<0.001) |
|
LS mean for percent change in TG at week 4 |
-15.66% |
3.73% |
−19.39% (P < 0.001) |
|
LS mean for percent change in TG at week 8 |
-9.38 |
7.56% |
−16.94% (p=0.0015) |
|
LS mean for percent change in HDL-C at week 4 |
16.3% |
4.71% |
11.59% (P = 0.001) |
|
LS mean for percent change in HDL-C at week 8 |
16.06% |
4.87% |
11.19% (P = 0.0008) |
- The incidence of treatment-emergent adverse events was slightly higher in the T/A+R group as compared with the T/A group, and the T+R group (17.91%, 14.93% and 12.31%, respectively). There were no adverse events leading to discontinuation or death during the study.
Conclusion
- Patient compliance while treating chronic diseases such as hypertension and dyslipidemia can be improved by reducing the number of pills and increasing patient convenience.
- Combined administration of telmisartan/amlodipine 80/5 mg and rosuvastatin 20 mg for the treatment of hypertensive patients with dyslipidemia significantly reduced blood pressure and improved lipid control.
- The combination may also contribute to the therapeutic goal of higher compliance over the long term due to ease of use and cost-effectiveness.
J Clin Hypertens. Sep 16, 2020 (Published Online); doi: 10.1111/jch.13893.










.webp?updated=20240527063817)