BPROAD Trial: Intensive vs. Standard SBP Control in Patients with Type 2 Diabetes Mellitus
Introduction
Patients affected with type-2 diabetes mellitus (T2DM) frequently have coexisting elevated systolic blood pressure (SBP). It is well established that reduction in BP, results in substantial reduction in the cardiovascular disease (CVD) risks. Nevertheless, the effective targets for SBP control in patients with T2DM are unclear.
Aim
The Blood Pressure Control Target in Diabetes (BPROAD) trial ascertained whether intensive treatment (target SBP <120 mmHg) would be more effective than standard treatment (target SBP <140 mmHg) in reducing the risk of major cardiovascular disease events (MACE) among patients with T2DM.
Patient Profile
- Patients with T2DM (aged ≥50 years, n=12821) with elevated SBP (130 to 180 mmHg in patients taking antihypertensive medications or ≥140 mmHg in patients not taking antihypertensive medications).
- All patients were at risk of CVD [defined as presence of at least one of the following: history of clinical CVD in prior 3 months, subclinical CVD in prior 3 years, 2/more CVD risk factors, and chronic kidney disease (CKD); estimated glomerular filtration rate (eGFR) 30-60 ml/min/1.73 m2 of body surface area]
Methods
Study Design
- A parallel-design, randomized clinical trial conducted across 145 clinical sites in China.
Treatment Strategy
- Patients were randomized 1:1 to receive intensive treatment (target SBP <120 mmHg, n=6414) or standard treatment (targeted SBP <140 mmHg, n=6407) for up to 5 years.
- Follow-up visits were conducted every month for the first 3 months and every 3 months thereafter to review for any drug adjustment if needed.
Outcomes
Primary Outcome
- A composite of the first occurrence of nonfatal stroke, nonfatal myocardial infarction (MI), treatment or hospitalization for heart failure (HF), or death from CV causes.
Secondary Outcomes
- Fatal or nonfatal stroke, fatal or nonfatal MI, treatment or hospitalization for HF, death from CV causes, death from any cause, and an expanded composite of the primary outcome or death from any cause.
CKD Outcomes
- Progression of CKD in patients with CKD at baseline (a composite of end-stage renal disease, an eGFR <15 ml/min/ 1.73 m2, or a >50% decrease in eGFR from baseline), development of CKD in patients without CKD at baseline (an eGFR <60 ml/min/1.73 m2 and a >30% decrease from baseline).
- Incident albuminuria [a doubling of the urinary albumin-to-creatinine ratio (UACR) from a value of <10 to a value of ≥10) in all patients with or without CKD.
Results
- The mean age of the study population was 63.8 years, 45.3% of the study population comprised of women. Approximately, 22.5% of the patients had a history of clinical CVD at baseline.
- Median follow-up period for the study was 4.2 years. The mean SBP at baseline was comparable in both the study groups (intensive group: 140.0 mmHg; standard group: 140.4 mmHg).
- At 1 year of follow-up, the mean SBP for patients in the intensive group was 121.6 mmHg (median,118.3 mm Hg) and that in the standard treatment group was133.2 mmHg (median, 135.0 mm Hg). Nearly 60% of the patients in the intensive treatment group met the SBP target.
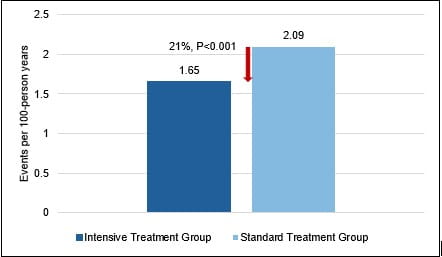
- The risk of primary-outcome events was 21% lower in the patients in the intensive treatment groups vs. those in the standard-treatment group [1.65 vs. 2.09 events per 100 person-years; hazard ratio (HR): 0.79; 95% confidence interval (CI), 0.69 to 0.90; P<0.001) (Fig. 1).
Fig. 1: Incidence of primary outcomes during the study
- The incidence of fatal or nonfatal stroke was 21% lower in the intensive treatment group vs. the standard treatment group. The incidence of other events including fatal or nonfatal MI, treatment or hospitalization for HF, and death from CV causes did not differ in the treatment groups (Table 1).
Table 1: Incidence of secondary outcomes in the study groups
Outcomes
Intensive Treatment Group (Number of events/ per 100 person yrs)
Standard Treatment Group
Hazard Rate (95% CI)
Fatal or nonfatal MI
0.28 (0.22–0.35)
0.33 (0.27–0.41)
0.84 (0.60–1.16)
Fatal or nonfatal stroke
1.19 (1.06–1.33)
1.50 (1.35–1.66)
0.79 (0.67–0.92)
Treatment or hospitalization for HF
0.13 (0.09–0.18)
0.19 (0.14–0.25)
0.66 (0.41–1.04)
Death from cardiovascular causes
0.24 (0.19–0.31)
0.32 (0.26–0.40)
0.76 (0.55–1.06)
Death from any cause
0.69 (0.59–0.80)
0.73 (0.63–0.84)
0.95 (0.77–1.17)
- The incidences of CKD progression and CKD development did not differ in the two study groups.
- The incidence of albuminuria was 11.29 events per 100 person-years) in the intensive-treatment group and 13.84 events per 100 person years in the standard-treatment group (HR:0.87; 95% CI, 0.77 to 0.97).
- The incidence of serious adverse events did not differ significantly in both the treatment groups. Nevertheless, the incidence of symptomatic hypotension and hyperkalemia was more frequent in the intensive-treatment group vs. the standard-treatment group.
Conclusions
- The BPROAD trial demonstrated that T2DM patients at high risk of CVD had a significant reduction in the incidence of MACE with intensive (<120 mmHg) vs. standard (<140 mmHg) SBP lowering.
N Engl J Med. 2025 Mar 27;392(12):1155-1167.