Asthma Symptom Control with Once-daily FF/VI 100/25 mcg versus Twice-daily B/F 160/4.5 mcg
Introduction
Fluticasone furoate/vilanterol (FF/VI) is an inhaled corticosteroids (ICS) / ultra LABA (long-acting ?2-agonist [LABA]) combination therapy licensed for the once-daily treatment of asthma in adults and adolescents aged ≥12 years in Europe and in adults aged ≥18 years in United States.
Aim
To compare asthma symptom control among adult patients who received once-daily FF/VI 100/25 mcg versus patients who received twice-daily budesonide/formoterol (B/F) 160/4.5 mcg
Patient Profile
Adult asthma patients
- ≥18 years of age on the index date
- ≥1 pharmacy claims for FF/VI 100/25 mcg or B/F 160/4.5 mcg
Methods
- Retrospective cohort study
- The study included propensity score (PS) matched adult asthma patients initiating once-daily FF/VI 100/25 mcg with patients initiating twice-daily (B/F) 160/4.5 mcg using a US claims database
- 51,537 were included in the B/F 160/4.5 mcg cohort
- Study duration: 12 months
- Asthma control was measured by the mean number of short-acting ?2-agonist (SABA) canisters dispensed per patient-year (PPY) during follow-up
Primary endpoint
Asthma control, as measured by the mean number of SABA canisters dispensed per patient-year (PPY)
Secondary Endpoint
- Time to first, rates of, overall and severe asthma exacerbations
Results
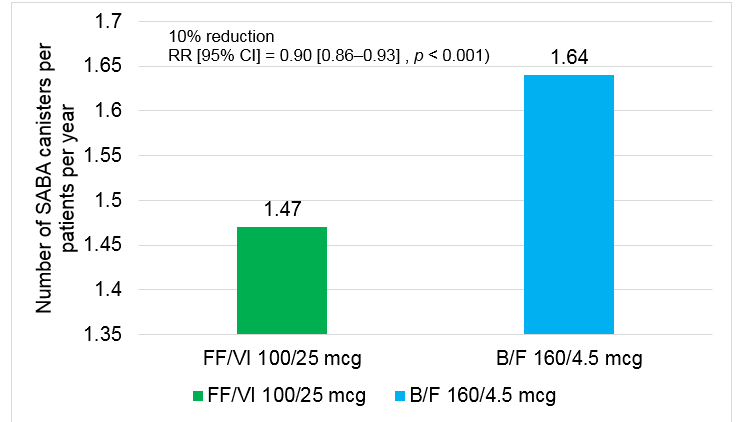
Asthma Control
- Mean SABA canisters dispensed PPY was significantly lower for FF/VI users compared with B/F users
- 10% reduction in use of SABA canisters was reported in participants who received FF/VI 100/25 mcg than those who received B/F 160/4.5 mcg
B/F, budesonide/formoterol; CI, confidence interval; FF/VI, fluticasone furoate/vilanterol; PPPY, per-patient per-year.
Secondary Endpoints
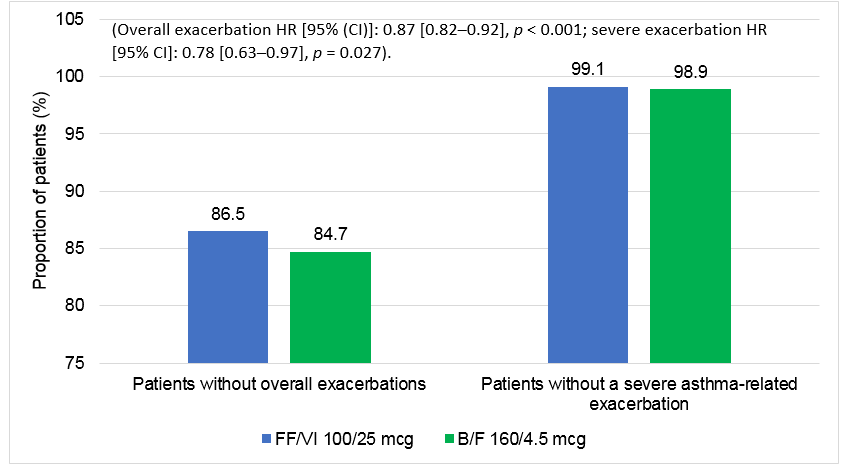
Time to first overall or severe asthma-related Exacerbation
- Time to first overall (moderate or severe) or severe asthma-related exacerbation was significantly longer in the FF/VI 100/25 mcg cohort relative to the B/F 160/4.5 mcg cohort at 3, 6, 9, and 12 months of follow-up (p < 0.05 at all-time points)
- FF/VI use resulted in 13% significantly lower risk of having an overall asthma-related exacerbation and 22% lower risk of a severe exacerbation versus B/F use
B/F, budesonide/formoterol; CI, confidence interval; FF/VI, fluticasone furoate/vilanterol
Rate of overall and severe asthma-related exacerbations
- Asthma-related exacerbation rates per 100 patient-days were a significantly lower for the FF/VI group compared with the B/F group (overall: 0.0475 vs. 0.0558, p < 0.001; severe: 0.0026 vs. 0.0033, p = 0.020)
- Initiation with FF/VI 100/25 mcg was also associated with a lower rate of overall (0.1796 vs. 0.2081) and severe (0.0097 vs. 0.0131) asthma-related exacerbations PPPY compared with initiation of B/F 160/4.5 mcg
Table 1: Overall and severe asthma-related exacerbations during follow-up in the main analysis
|
|
Rate (PPPY) |
Rate (per 100 patient-days) |
Rate ratio (95% CI) |
p-value | ||
|
Asthma-related exacerbations |
FF/VI (100/25 mcg) |
B/F (160/4.5 mcg) |
FF/VI (100/25 mcg) [A] |
B/F (160/4.5 mcg) [B] |
[A] / [B] |
|
|
Overall |
0.1796 |
0.2081 |
0.0475 |
0.0558 |
0.85 (0.81–0.91) |
<0.001 |
|
Severe |
0.0097 |
0.0131 |
0.0026 |
0.0033 |
0.78 (0.62–0.96) |
0.020 |
B/F, budesonide/formoterol; CI, confidence interval; FF/VI, fluticasone furoate/vilanterol; PPPY, per-patient per-year
Subgroup Analysis (patient with >1 prior exacerbations)
- Significantly fewer SABA canisters were used in patients with ≥1 prior exacerbation (overall or severe) who were initiated on FF/VI 100/25 mcg during the follow-up period (1.75 vs. 2.06 PPY; RR [95% CI] = 0.85 [0.78–0.92], p < 0.001)
- 15% reduction in use of SABA canisters was reported in patients with ≥1 prior exacerbation receiving FF/VI 100/25 mcg compared to patients who received B/F 160/4.5 mcg
- Significantly longer time to first overall (moderate or severe) or severe asthma-related exacerbation was observed (p < 0.05)
- 21% lower risk of overall asthma-related exacerbations (HR [95% CI] = 0.79 [0.71–0.88], p < 0.001) and a 32% lower risk of severe asthma-related exacerbations at 12 months post-index was reported (HR [95% CI] = 0.68 [0.47–0.99], p = 0.047) than patients in the B/F 160/4.5 mcg cohort
- FF/VI 100/25 mcg was associated with a significantly lower rate of overall (RR [95% CI] = 0.80 [0.72–0.90]; p < 0.001) and severe (RR [95% CI] = 0.62 [0.42–0.98]; p = 0.028) asthma-related exacerbations per 100 patient-days compared with initiation of B/F 160/4.5 mcg
- Initiation with FF/VI 100/25 mcg was associated with a lower rate of overall (0.3361 vs 0.4103) and severe (0.0189 vs 0.0332) asthma-related exacerbations compared with initiation of B/F 160/4.5 mcg
Conclusion
The study demonstrated that in real-world practice, initiation of once-daily FF/VI 100/25 mcg in adults with asthma was associated with significantly lower use of SABA and fewer asthma-related exacerbations, which may indicate better asthma control, when compared with use of twice-daily B/F 160/4.5 mcg.
Reference
J Asthma. 2021 Aug 25:1-14. doi: 10.1080/02770903.2021.1963767.