As-needed Budesonide-Formoterol Effective and Safe in Adolescents with Mild Asthma: Pooled Analysis of SYGMA Studies
Introduction
Low-dose inhaled corticosteroids (ICS) brings about effective symptom control and reduces the risk of exacerbations in asthma including mild asthma. However, poor adherence to medications and over-reliance on short acting ?-agonists (SABA) have been the major challenges in the management of asthma, especially for adolescents. The results of SYGMA 1 trial concluded that treatment with as-needed budesonide-formoterol (BUD-FORM) reduces the severe exacerbations as compared to as-needed terbutaline, with similar efficacy to BUD maintenance. The severe exacerbation rates over 12 months were similar between as-needed BUD-FORM and BUD maintenance, as per the findings of SYGMA 2 trial.
Aim
This post hoc pooled analysis of SYGMA 1 and 2 evaluated the efficacy, medication adherence and safety of as-needed BUD-FORM in adolescents with mild asthma.
Patient Profile
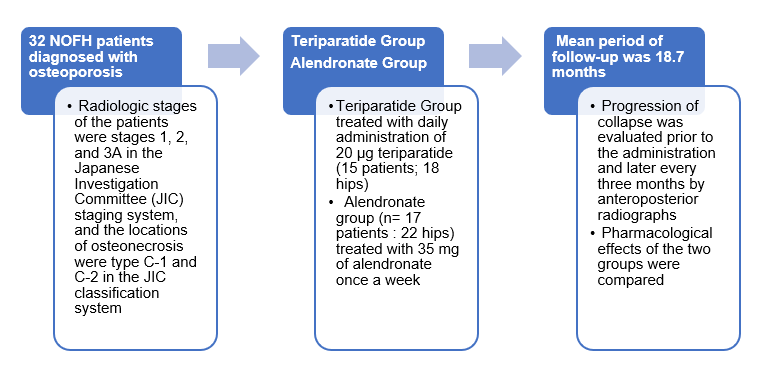
- Subjects aged >12 years with mild asthma either uncontrolled with as-needed inhaled short-acting bronchodilators or controlled on low-dose ICS or LTRA.
Method
Study Design
- Post-hoc analysis of two randomized, double-blind, parallel-group, 52-week phase 3 studies SYGMA 1 and SYGMA 2
Treatment Strategy
- Patients were randomized into 3 arms
- Twice-daily placebo + as-needed BUD-FORM 200/6 ?g
- Twice-daily BUD 200 ?g + as-needed terbutaline (BUD maintenance)
- Twice-daily placebo + as-needed terbutaline 0.5 mg (SYGMA 1 only).
End Points
- Annualized severe exacerbation rates
- Time to first severe exacerbation
- Pulmonary function in terms of forced expiratory volume in 1 sec % predicted (%FEV1)
- Asthma symptom control using Asthma Control Questionnaire (ACQ-5).
- Adherence to randomized maintenance treatment
- Adverse events (AEs)
- Severe AEs (SAEs)
Results
- The overall cohort comprised of 889 adolescents: 478 from SYGMA 1 and 411 from SYGMA 2.
- In SYGMA 1, annualized severe exacerbation rate was 77% lower with as-needed BUD-FORM than as-needed terbutaline (p=0.005) as seen in Figure 1.
- The results of pooled analysis revealed no significant difference in the annual rate of severe exacerbations in BUD-FORM group vs BUD maintenance group; 0.08 vs 0.07 per patient per year (p=0.634).
- Time to first severe exacerbation was significantly longer in BUD-FORM group as compared to as-needed terbutaline; hazard ratio (HR) 0.33; p=0.02.
- There was no significant difference in the time to first severe exacerbation in both the groups in pooled analysis; HR 1.23; p=0.47.
- The mean ACQ-5 score improved from baseline across all the 3 groups and the difference was not significant.
- A significant difference in change in FEV1 % predicted from baseline was demonstrated with as-needed BUD-FORM versus BUD maintenance in SYGMA 1 (estimated difference, −3.9%; p < .001) and in the pooled analysis (estimated difference, −2.3%; p < .001), but not in SYGMA 2
- The overall median adherence to medication was 73% and 51% in SYGMA 1 and SYGMA 2 respectively.
- The adolescents aged ≥12 years to <14 years in the BUD-FORM group had significantly greater change in height from baseline as compared to BUD maintenance (4.8 vs 3.9 cm); p < 0.046.
- In SYGMA 1, the mean change in height of adolescents was similar between as-needed BUD-FORM and as-needed terbutaline (4.5 cm vs 4.1 cm); p = 0.500.
- The incidence of AEs was similar in BUD-FORM and BUD-maintenance groups and higher in as-needed terbutaline group as seen in Figure 2.
- SAEs were also slightly higher in as-needed terbutaline group.
- There were no new or unexpected safety concerns that were identified in this pooled analysis.
Conclusions
- Reliever therapy with as-needed budesonide-formoterol (BUD-FORM) was superior to as-needed terbutaline and significantly reduced the rate of severe exacerbations and demonstrated similar efficacy to BUD maintenance in terms of symptom control and lung function in adolescents with mild asthma.
- As-needed BUD-FORM can be considered as an ideal alternative in adolescents with mild asthma, which can avoid daily maintenance treatment.
J Allergy Clin Immunol Pract. 2021 Apr 22; S2213-2198(21)00456-6. Doi: 10.1016/j.jaip.2021.04.016.